research
研究内容
Prof. Dr. Mori
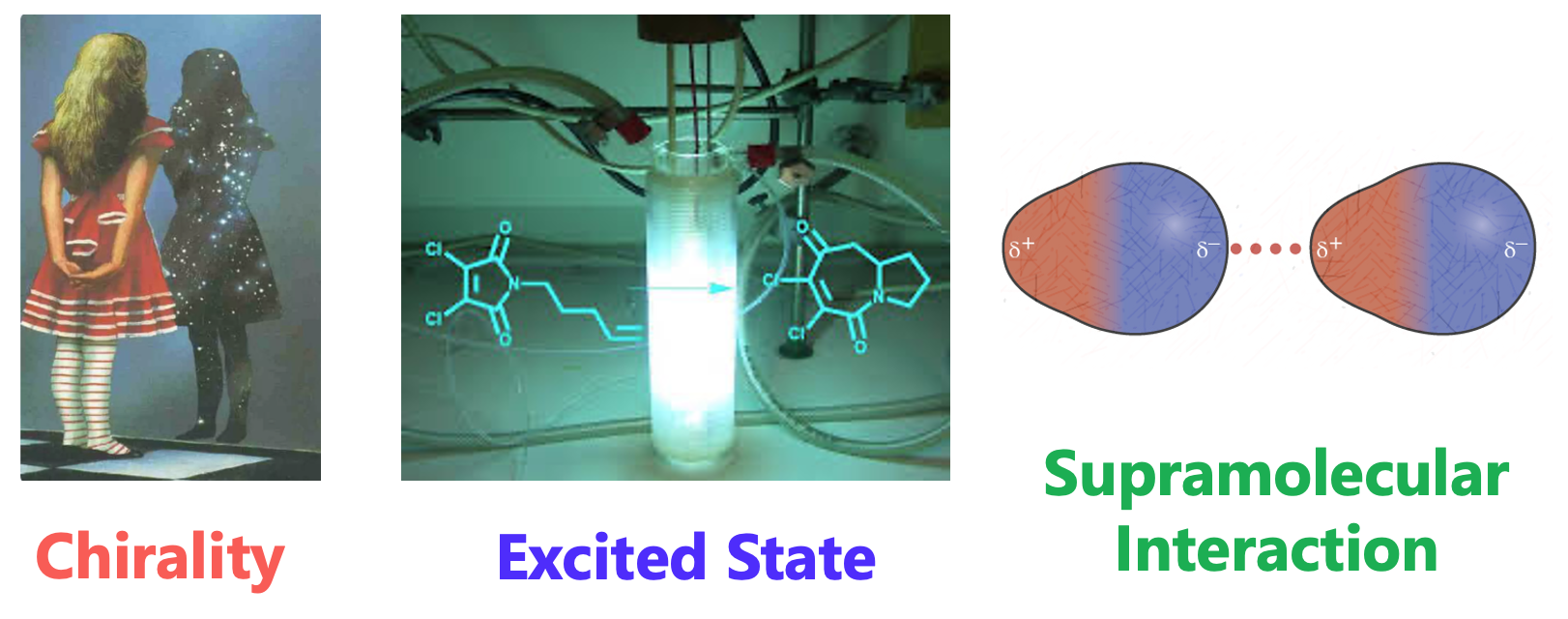
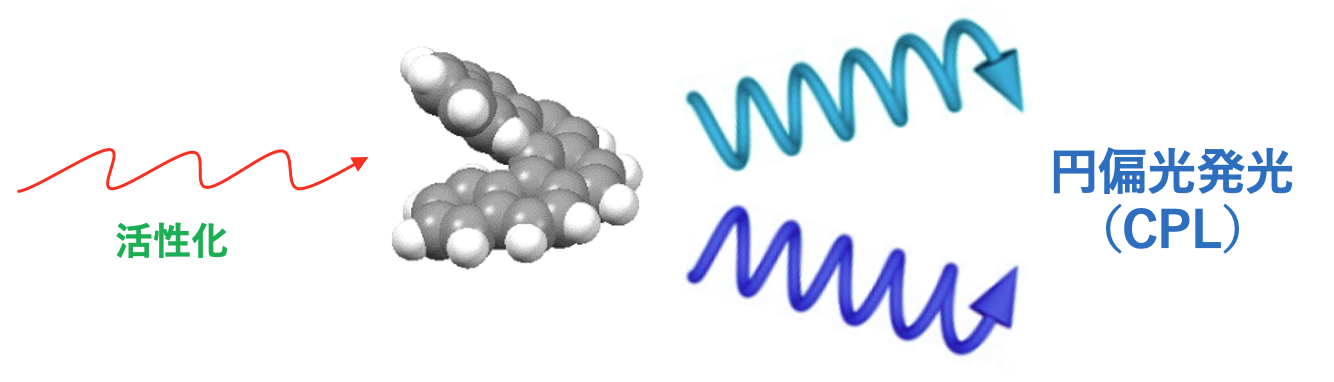
励起状態キラリティー
Excited State Chirality
励起状態キラリティーは,光反応および光物理において極めて重要な役割を果たします.従来の方法に加え,超分子相互作用を含む新しい戦略を通じて,非対称光反応の制御を可能にします.さらに,円二色性や円偏光発光といった光学的性質を,材料科学の観点から構造―物性相関として解明することも含まれます。これを実現するためには,分子の配座,エネルギー,動的挙動や諸物性に関する精密な励起状態計算が不可欠であり,加えて人間の直観(ときに常識にとらわれない直観)が大きな力を発揮します.
This concept plays a crucial role in photoreactions and photophysics. It encompasses the control of asymmetric photoreactions through both conventional and emerging strategies, including supramolecular interactions. Additionally, it involves elucidating the structure-property relationships of circular dichroism and circularly polarized luminescence from a materials perspective. Achieving this requires highly accurate excited state computations regarding conformation, energy, dynamics, and property calculations, along with human (and sometimes unconventional) intuition.
Prof. Dr. Mori
キラル発光と不斉光反応
Excited State Chirality
🏗️工事中🚧
Dr. Tsunoi
質量分析計は,構造解析・分子量の決定に利用される.また,質量分析計はGCやLCなどの分離装置と組み合わせることによって,高感度な検出器として用いられることも多い.質量分析は構造解析によく使われるが,異性体の質量スペクトルは非常によく似ており,異性体をマススペクトルで識別するのは困難である.GC-MSやLC-MSでも異性体の識別はタンデム質量分析を用いたり,保持時間で識別したりすることが多い.
通常,GC-MSのイオン化には電子イオン化や化学イオン化が用いられる.化学イオン化質量分析(CI-MS)はメタン,イソブタン,アンモニアなどの試薬ガスを使って分析対象物質をイオン化する.我々は,試薬ガスとしてケイ素化合物を使い,一般に識別が困難な異性体の分析を行っている.ケイ素化合物から生成するケイ素カチオン特有の反応,また付加イオンのタンデム質量分析により薬物異性体の識別を検討している.
Mass spectrometers are used for structural analysis and determination of molecular weight. They are also often used as highly sensitive detectors when combined with separation devices such as GC or LC. Mass spectrometry is commonly used for structural analysis, but the mass spectra of isomers are very similar, making it difficult to discriminate them. Even with GC-MS and LC-MS, isomers are often discriminated using tandem mass spectrometry or retention time.
Typically, electron ionization or chemical ionization are used for ionization in GC-MS. Chemical ionization mass spectrometry (CI-MS) typically uses reagent gases such as methane, isobutane, and ammonia to ionize the analyte. We use silicon compounds as reagent gases to analyze isomers that are generally difficult to discriminate. We are investigating the differentiation of drug isomers using the specific reactions of silicon cations produced from silicon compounds and tandem mass spectrometry of the adduct ions.
Dr. Wang
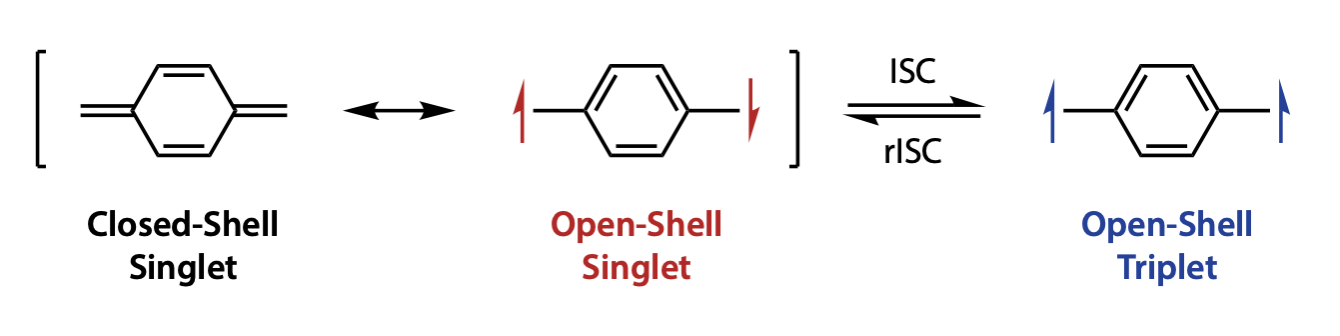
溶解性と化学安定性を両立した開殻ジラジカロイドの創製と応用展開
Creation of Open-Shell Diradicaloids with Balanced Solubility and Chemical Stability
分子内に不対電子をもつ化学種は開殻種と呼ばれ,開殻性ゆえに一般に熱的・化学的に不安定で反応性が高く,合成や取り扱いが難しいという課題があります.ジラジカル(ジラジカロイド)はしばしば HOMO–LUMO ギャップが小さく,長波長光の吸収や多段階の酸化還元を示すため,光学・有機電子材料としての高い潜在力から注目を集めています.近年,特定の多環炭化水素において基底状態での一重項開殻ジラジカル性,さらにはポリラジカル性が見いだされ,特異な電子・光学・磁気特性の発現が報告されています.
私たちは,溶解性と化学安定性を両立させた機能性ジラジカロイドの創製を目指しています.これらの研究を通じて,スピンの局在/非局在が電子特性を本質的に左右することを明らかにし,得られたジラジカロイド/ポリラジカロイドがエレクトロニクス,フォトニクス,スピントロニクス,磁性材料,量子情報処理などへの応用が期待されています.
Chemical species bearing unpaired electrons within a molecule are referred to as open-shell species. Owing to their open-shell nature, they are generally thermally and chemically unstable and highly reactive, which makes their synthesis and handling challenging. Diradicals (diradicaloids) often possess small HOMO–LUMO gaps and therefore exhibit long-wavelength absorption and multistep redox behavior, attracting considerable attention for their high potential as optical and organic electronic materials. In recent years, certain polycyclic aromatic hydrocarbons have been found to display ground-state singlet open-shell diradical character—and even polyradical character—giving rise to unique electronic, optical, and magnetic properties.
We aim to develop functional diradicaloids that simultaneously achieve good solubility and chemical stability. Through these studies, we are elucidating how the degree of spin localization/delocalization fundamentally governs electronic properties, and the resulting diradicaloids/polyradicaloids are expected to find applications in electronics, photonics, spintronics, magnetic materials, and quantum information processing.
Dr. Wang
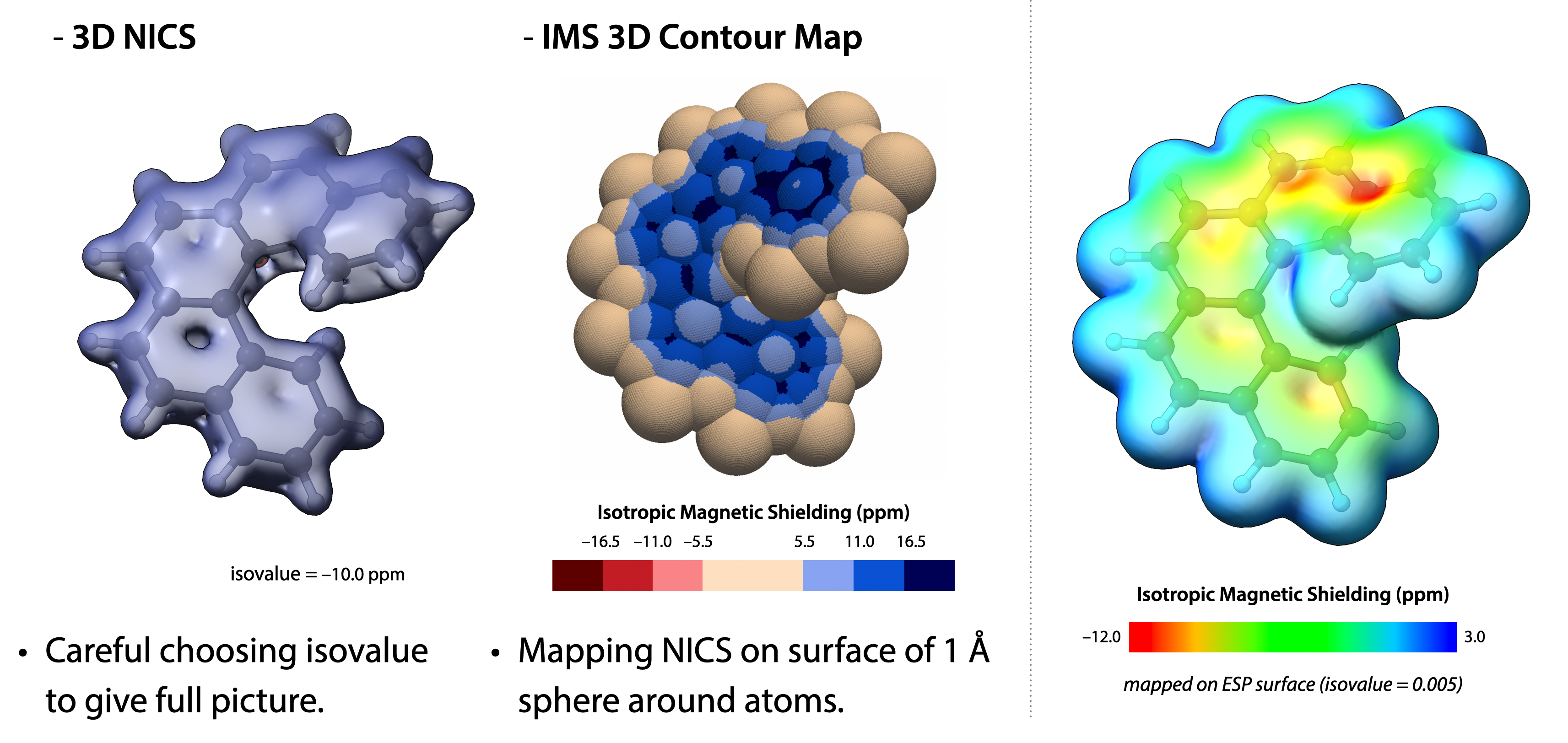
芳香族性評価の計算化学的手法の改良
Advancing Computational Methods for the Evaluation of Aromaticity
芳香族性は環状分子の安定性・反応性を示唆する基盤概念であり,直接観測が難しいため,構造・エネルギー・反応性・磁気的性質など多面的な指標で評価されてきました.中でも核独立化学シフト(NICS)は手軽さから広く普及していますが,評価点の置き方や座標系・参照面の選択に依存して結果がばらつく,すなわち「全ての系に常に適切とは言い切れない」という弱点が指摘されています.私たちは,この一般的な限界を超えるために「平面・非平面・傾斜・縮環・置換系」など多様な分子で一貫性と再現性の高い芳香族性評価が可能な手法の開発を目指しています.
Aromaticity is a fundamental concept that indicates the stability and reactivity of cyclic molecules. Since it is difficult to observe directly, it has been evaluated using multiple criteria, including structural, energetic, reactivity-based, and magnetic properties. Among these, the nucleus-independent chemical shift (NICS) has become the most widely adopted method due to its simplicity. However, its results are known to vary depending on the choice of evaluation points, coordinate system, and reference plane, meaning that it is not always universally appropriate for all molecular systems. To overcome these inherent limitations, this study aims to develop a methodology that enables consistent and reproducible evaluation of aromaticity across diverse molecules, including planar, nonplanar, tilted, fused, and substituted systems.