accounts & reviews
all / original papers / accounts & reviews / cover pictures
-
 Small molecule helical emittersTadashi Mori*Chem. Soc. Rev., 2026, Advanced Article.
Small molecule helical emittersTadashi Mori*Chem. Soc. Rev., 2026, Advanced Article.The development of materials exhibiting circularly polarized luminescence (CPL) is a key area of research for next-generation optical technologies, including 3D displays and secure communications. The central goal in this field is to create chiral emitters with a high luminescence dissymmetry (gCPL) factor, a measure of the emission’s chirality. While theoretically reaching ±2, practical values in small organic molecules have historically been much lower, on the order of 0.001 or less. This summary outlines the core strategies in molecular design focusing on helical emitters that have recently enabled significant breakthroughs, pushing g values beyond the 0.01 threshold. The magnitude of g factor is determined by the cosine of the angle between the electric (μe) and magnetic (μm) dipole transition moments, as well as their respective magnitudes. Consequently, the most successful research has moved beyond simple screening and has focused on rationally engineering molecules to optimize this relationship. One of the most direct strategies has been to design rigid, helical molecules where high symmetry forces the μe and μm to be parallel. By enforcing D2 and other symmetry in certain helicenes, helical nanographenes and related structures, researchers have minimized the angle between the moments, thus maximizing the cosine term and leading to a significant enhancement in the g factor value. A second, distinct approach targets the magnitude of the μm. In most organic chromophores, μm is inherently small, limiting the potential g factor intensity. To overcome this, researchers have designed for example belt-shaped macrocyclic molecules that function as molecular-scale solenoids. The cyclic arrangement of chromophores induces a large, circulating electric current in the excited state, which in turn generates a powerful μm along the cylinder’s axis. A third innovative strategy circumvents the limitation of a small intrinsic μm by leveraging exciton coupling between two and more chromophores. In these systems, two π-conjugated units such as pyrene are held in a fixed, chiral arrangement. Upon photoexcitation, they form an intramolecular excimer, a transient excited-state complex with a well-defined helical geometry. The resulting CPL signal originates from the chiral interaction of the two strong electric transition moments, generating a large rotational strength and a high g factor without relying on the weak magnetic moment of the individual units. The progress in CPL-active materials is a testament to the power of targeted molecular engineering. As seen in the state-of-the-art examples in the review, the field has matured to a point where the fundamental photophysical principles governing CPL are being directly translated into synthetic molecular designs. While current high-performing materials are often complex and synthetically challenging, these proof-of-concept molecules validate the core design strategies.
@article{mori2026small, title = {Small molecule helical emitters}, author = {Mori, Tadashi}, journal = {Chem. Soc. Rev.}, pages = {Advanced Article}, year = {2026}, publisher = {Royal Society of Chemistry}, doi = {10.1039/d5cs01270h}, url = {https://doi.org/10.1039/d5cs01270h}, dimensions = {true}, tab = {review}, } -
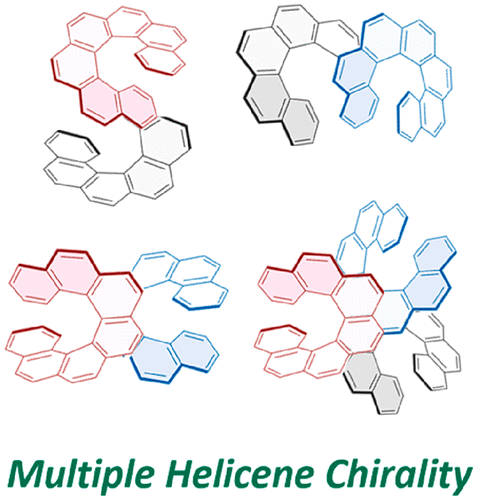 Chiroptical properties of symmetric double, triple, and multiple helicenesTadashi Mori*Chem. Rev., 2021, 121, 2373–2412.
Chiroptical properties of symmetric double, triple, and multiple helicenesTadashi Mori*Chem. Rev., 2021, 121, 2373–2412.Helicenes have attracted considerable attention due to their inherent helical chirality and extended π-conjugation. Recently, rapid progress has been witnessed in the preparation of double, triple, quadruple, quintuple, and sextuple helicenes, where plural helicene moieties are symmetrically arranged in a single molecule. While synthetic efforts and X-ray crystallographic analyses devoted to these multiple helicenes and theoretical investigations on their isomerization and racemization behaviors have been relatively well documented and reviewed in the literature, the chiroptical properties of the multiple helicities have been somewhat overlooked. This review discourses the cumulative and systematic investigations on the chiroptical properties such as the circular dichroism (CD) and circularly polarized luminescence (CPL) of multiple helicenes. Although the number and structural variations of multiple helicenes reported to date have been fairly limited, this review overviews the current status of the chemistry of multiple helicenes from the viewpoint of chiroptical properties and provides insights into the design principle for advanced chiroptical materials through the proper arrangement of multiple helices, highlighting the impact of the molecular symmetry on the chiroptical responses.
@article{mori2021chiroptical, title = {Chiroptical properties of symmetric double, triple, and multiple helicenes}, author = {Mori, Tadashi}, journal = {Chem. Rev.}, volume = {121}, issue = {4}, pages = {2373--2412}, year = {2021}, publisher = {ACS Publications}, doi = {10.1021/acs.chemrev.0c01017}, url = {https://doi.org/10.1021/acs.chemrev.0c01017}, dimensions = {true}, tab = {review}, } -
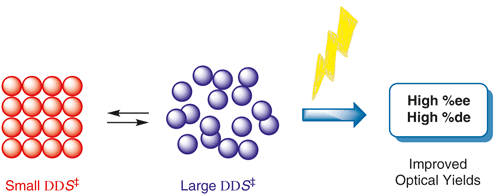 Relevance of the entropy factor in stereoselectivity control of asymmetric photoreactionsTadashi Mori*Synlett, 2020, 31, 1259–1267.
Relevance of the entropy factor in stereoselectivity control of asymmetric photoreactionsTadashi Mori*Synlett, 2020, 31, 1259–1267.Entropy as well as enthalpy factors play substantial roles in various chemical phenomena such as equilibrium and reactions. However, the entropy factors are frequently underestimated in most instances, particularly in synthetic chemistry. In reality, the entropy factor can be in competition with the enthalpy factor or can even be decisive in determining the overall free or activation energy change upon molecular interaction and chemical transformation, particularly where weak interactions in ground and/or excited states are significant. In this account, we overview the importance of the entropy factor in various chemical phenomena in both thermodynamics and kinetics and in the ground and excited states. It is immediately apparent that many diastereo- and enantioselective photoreactions are entropy-controlled. Recent advances on the entropy-control concept in asymmetric photoreactions are further discussed. Understanding the entropy-control concept will pave the way to improve, fine-tune, and even invert the chemo- and stereoselectivity of relevant chemical phenomena.
@article{mori2020relevance, title = {Relevance of the entropy factor in stereoselectivity control of asymmetric photoreactions}, author = {Mori, Tadashi}, journal = {Synlett}, volume = {31}, issue = {13}, pages = {1259--1267}, year = {2020}, publisher = {Georg Thieme Verlag}, doi = {10.1055/s-0040-1707962}, url = {https://doi.org/10.1055/s-0040-1707962}, dimensions = {true}, tab = {review}, } -
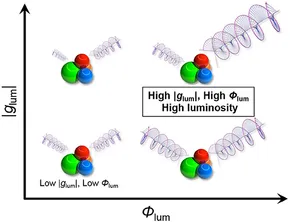 Irreverent nature of dissymmetry factor and quantum yield in circularly polarized luminescence of small organic moleculesYuya Nagata and Tadashi Mori*Front. Chem., 2020, 8, 448.
Irreverent nature of dissymmetry factor and quantum yield in circularly polarized luminescence of small organic moleculesYuya Nagata and Tadashi Mori*Front. Chem., 2020, 8, 448.Recently, a rational modification of small organic molecules has attracted considerable attention for designing advanced materials with enhanced circularly polarized luminescence (CPL) activity. A particular emphasis has been placed on fully allowed π-π* transition of rigid aromatic systems, due to their relatively superior emission properties or quantum yields of luminescence (Φlum). However, their dissymmetry factors (glum), differential left and right CPL intensities, are typically disappointingly low at least in one to two orders of magnitude. Truly useful organic CPL materials, rated by a circular polarization luminosity index (ΛCPL) per single molecule, possess both |glum| and Φlum values high. However, how to improve these two factors simultaneously with a proper molecular design is an open question. Here, we addressed this issue by theoretical and statistical inspection on a possible relation of the glum and Φlum values. According to the analysis, we propose simple, unpretentious, yet pertinent guidelines for designing superior organic CPL materials for the future with large ΛCPL values.
@article{nagata2020irreverent, title = {Irreverent nature of dissymmetry factor and quantum yield in circularly polarized luminescence of small organic molecules}, author = {Nagata, Yuya and Mori, Tadashi}, journal = {Front. Chem.}, volume = {8}, pages = {448}, year = {2020}, publisher = {Frontiers Media SA}, doi = {10.3389/fchem.2020.00448}, url = {https://doi.org/10.3389/fchem.2020.00448}, dimensions = {true}, tab = {review}, } -
 Circularly polarized luminescence and circular dichroisms in small organic molecules: correlation between excitation and emission dissymmetry factorsHiroki Tanaka, Yoshihisa Inoue, and Tadashi Mori*ChemPhotoChem, 2018, 2, 386–402.
Circularly polarized luminescence and circular dichroisms in small organic molecules: correlation between excitation and emission dissymmetry factorsHiroki Tanaka, Yoshihisa Inoue, and Tadashi Mori*ChemPhotoChem, 2018, 2, 386–402.Prompted by the recent rapid growth of interest in circularly polarized luminescence (CPL) of organic molecules, we have collected all the reliable CPL, as well as the corresponding circular dichroism (CD), data measured in fluid solutions. To analyze the correlation between CPL and CD, we employed the absorption and luminescence dissymmetry factors (gabs and glum) of the π–π* transition reported for chiral organic molecules of various categories, including planar chiral cyclophanes and helicenes, axially chiral biaryls and spiro compounds, and point- and axially chiral BODIPY derivatives. In rigid π-systems, the absorption and fluorescence spectra are often mirror images of each other with a small Stokes shift, reflecting the minimal conformational relaxation in the emissive excited state, which should also affect the chiroptical properties in the excited state and be better sensed by CPL. However, no comprehensive efforts have hitherto been made to correlate the two relevant chiroptical properties, i.e. CPL versus CD, and also to quantitatively elucidate the effects of conformational relaxation in the excited state on the CPL behavior. The global linear regression analysis of all the reported gabs and glum values, though fairly scattered (see TOC), led us to a quantitative relationship: |glum|=0.81×|gabs| (r2=0.60), which demonstrates that the CPL dissymmetry factor is proportional to, and smaller than, the CD dissymmetry factor. A closer look revealed that the slope of the plot, or the proportional coefficient, is a critical function of the class of compounds, varying from 0.99 for cyclophanes to 0.93 for biaryls, to 0.77 for BODIPYs, and then to 0.61 for helicenes/helicenoids. The scattered glum–gabs plot and the general trend glum≤gabs appear to be inherent to the CPL of organic molecules in their isolated states, originating from the conformational flexibility, vibrational contribution, and Stokes shift that differ in each category.
@article{tanaka2018circularly, title = {Circularly polarized luminescence and circular dichroisms in small organic molecules: correlation between excitation and emission dissymmetry factors}, author = {Tanaka, Hiroki and Inoue, Yoshihisa and Mori, Tadashi}, journal = {ChemPhotoChem}, volume = {2}, issue = {5}, pages = {386--402}, year = {2018}, publisher = {Wiley Online Library}, doi = {10.1002/cptc.201800015}, url = {https://doi.org/10.1002/cptc.201800015}, dimensions = {true}, tab = {review}, } -
 Asymmetric Photochemical Synthesis Based on Selective Excitation of Charge-Transfer ComplexesTadashi Mori*J. Syn. Org. Chem., Jpn., 2017, 75, 144–152.
Asymmetric Photochemical Synthesis Based on Selective Excitation of Charge-Transfer ComplexesTadashi Mori*J. Syn. Org. Chem., Jpn., 2017, 75, 144–152.The use of photoreactions such as photoredox catalysts in asymmetric synthesis has been expansively recognized as expedient procedure over the past decade. In this paper, a potential application of photoreaction of Charge-Transfer (CT) complex for asymmetric synthesis is described. In chiral donor-acceptor systems, excitation wavelength, along with other environmental factors such as solvent polarity and temperature, plays more substantial role in determining the stereoselectivity of the photochemical outcomes. Accordingly, the excited CT complex produced upon the selective CT-band excitation behaves completely different from the conventional exciplex, which is formed by the direct excitation of donor or acceptor. Such unique wavelength effect, together with other entropic controls, may possibly come to be valuable means in asymmetric photochemical synthesis for the future.
@article{mori2017asymmetric, title = {Asymmetric Photochemical Synthesis Based on Selective Excitation of Charge-Transfer Complexes}, author = {Mori, Tadashi}, journal = {J. Syn. Org. Chem., Jpn.}, volume = {75}, number = {2}, pages = {144--152}, year = {2017}, doi = {10.5059/yukigoseikyokaishi.75.144}, url = {https://doi.org/10.5059/yukigoseikyokaishi.75.144}, dimensions = {true}, tab = {review}, } - Entropy Control of ReactionsTadashi MoriJ. Syn. Org. Chem., Jpn., 2017, 75, 160.
@article{mori2017entropy, title = {Entropy Control of Reactions}, author = {Mori, Tadashi}, journal = {J. Syn. Org. Chem., Jpn.}, volume = {75}, issue = {2}, pages = {160}, year = {2017}, doi = {10.5059/yukigoseikyokaishi.75.160}, url = {https://doi.org/10.5059/yukigoseikyokaishi.75.160}, dimensions = {true}, tab = {review} } -
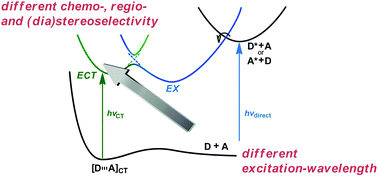 Charge-transfer excitation: unconventional yet practical means for controlling stereoselectivity in asymmetric photoreactionsTadashi Mori* and Yoshihisa InoueChem. Soc. Rev., 2013, 42, 8122–8133.
Charge-transfer excitation: unconventional yet practical means for controlling stereoselectivity in asymmetric photoreactionsTadashi Mori* and Yoshihisa InoueChem. Soc. Rev., 2013, 42, 8122–8133.In chiral donor–acceptor (D–A) systems, irradiation wavelength plays vital roles in determining the photochemical consequences. Selective excitation of a D–A complex at the charge-transfer (C-T) band affords an excited C-T complex (ECT), while the local-band excitation of D or A may lead to the formation of a conventional exciplex (EX) upon subsequent interaction with the D–A partner. These two excited species, generated from the same D–A pair, may be categorized formally as excited complexes or exciplexes, but should be distinguished, provided that they significantly differ in structure and reactivity. Indeed, ECT and EX exhibit distinctly different temperature-dependent photophysical and photochemical behaviours, which are assignable to the differences in relative stability, conformational flexibility and/or solvation properties. Fine-tuning excitation wavelength further enabled us to discriminate stereoisomeric intramolecular C-T complexes through preferential excitation, as C-T complexes are generally composed of an ensemble of various geometries. Besides temperature and solvent polarity, the excitation wavelength was shown to be employed as an unconventional yet practical tool for critically controlling the chemo-, regio- and stereoselectivities in molecular and supramolecular photochemistry.
@article{mori2013charge, title = {Charge-transfer excitation: unconventional yet practical means for controlling stereoselectivity in asymmetric photoreactions}, author = {Mori, Tadashi and Inoue, Yoshihisa}, journal = {Chem. Soc. Rev.}, volume = {42}, number = {20}, pages = {8122--8133}, year = {2013}, publisher = {Royal Society of Chemistry}, doi = {10.1039/C3CS60117J}, url = {https://doi.org/10.1039/C3CS60117J}, dimensions = {true}, tab = {review}, } -
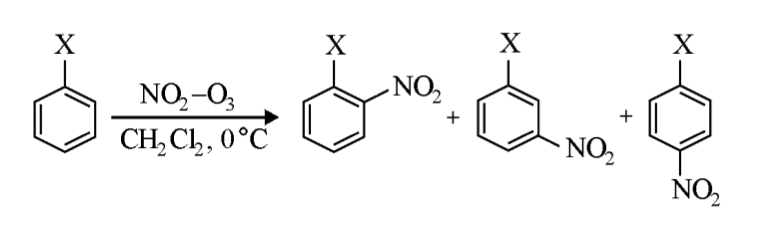 Kyodai-Nitration. An Alternative Electrophilic Route to Aromatic Nitro Compounds Based on Nitrogen TrioxideNobuaki Nonoyama, Tadashi Mori, and Hitomi Suzuki*Russ. J. Org. Chem., 1998, 34, 1521–1531.
Kyodai-Nitration. An Alternative Electrophilic Route to Aromatic Nitro Compounds Based on Nitrogen TrioxideNobuaki Nonoyama, Tadashi Mori, and Hitomi Suzuki*Russ. J. Org. Chem., 1998, 34, 1521–1531.@article{nonoyama1998kyodai, title = {Kyodai-Nitration. An Alternative Electrophilic Route to Aromatic Nitro Compounds Based on Nitrogen Trioxide}, author = {Nonoyama, Nobuaki and Mori, Tadashi and Suzuki, Hitomi}, journal = {Russ. J. Org. Chem.}, volume = {34}, issue = {11}, pages = {1521--1531}, year = {1998}, publisher = {Kluwer Academic Plenum Publisher}, doi = {10.1002/chin.199917310}, url = {https://doi.org/10.1002/chin.199917310}, dimensions = {true}, tab = {review}, } -
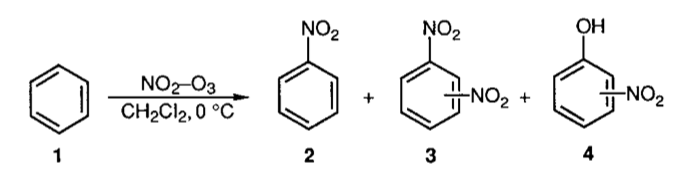 Ozone-mediated Nitration of Aromatic Compounds with Lower Oxides of Nitrogen (The Kyodai-Nitration)Tadashi Mori and Hitomi Suzuki*Synlett, 1995, 34, 383–392.
Ozone-mediated Nitration of Aromatic Compounds with Lower Oxides of Nitrogen (The Kyodai-Nitration)Tadashi Mori and Hitomi Suzuki*Synlett, 1995, 34, 383–392.A novel non-acid methodology for the preparative nitration of aromatic compounds with the lower oxides of nitrogen using a combination of ozonized oxygen or air and some third substance as the promoter is described. The reaction, referred to as the kyodai-nitration, is now subject to industrially based research as the promising pollution-free and energy-saving substitute for the century-old, yet currently ongoing commercial process based on the use of nitric acid-sulfuric acid.
@article{mori1995ozone, title = {Ozone-mediated Nitration of Aromatic Compounds with Lower Oxides of Nitrogen (The Kyodai-Nitration)}, author = {Mori, Tadashi and Suzuki, Hitomi}, journal = {Synlett}, number = {5}, pages = {383--392}, year = {1995}, publisher = {Thieme}, doi = {10.1055/s-1995-4979}, url = {https://doi.org/10.1055/s-1995-4979}, dimensions = {true}, tab = {review}, }