articles in 2022
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
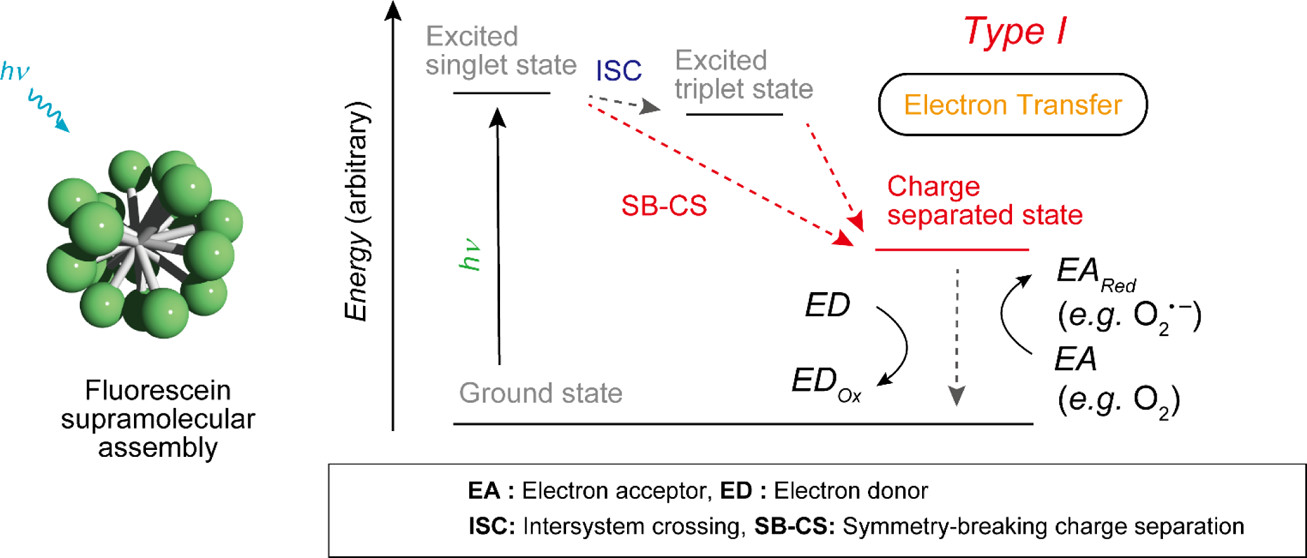 Fluorescein-Based Type I Supramolecular Photosensitizer via Induction of Charge Separation by Self-AssemblyHajime Shigemitsu*, Kei Ohkubo, Kazuhide Sato, Asuka Bunno, Tadashi Mori, Yasuko Osakada, Mamoru Fujitsuka, and Toshiyuki Kida*JACS Au, 2022, 2, 1472–1478.
Fluorescein-Based Type I Supramolecular Photosensitizer via Induction of Charge Separation by Self-AssemblyHajime Shigemitsu*, Kei Ohkubo, Kazuhide Sato, Asuka Bunno, Tadashi Mori, Yasuko Osakada, Mamoru Fujitsuka, and Toshiyuki Kida*JACS Au, 2022, 2, 1472–1478.Photosensitizers (PSs) are critical substances with considerable potential for use in non-invasive photomedicine. Type I PSs, which generate reactive radical species by electron transfer from the excited state induced via photoirradiation, attracted much attention because of their suitability for photodynamic therapy (PDT) irrespective of the oxygen concentration. However, most organic PSs are type II, which activates only oxygen, generating singlet oxygen (1O2) via energy transfer from the triplet state. Here, we proposed a strategy to form type I supramolecular PSs (SPSs) utilizing the charge-separated state induced by self-assembly. This was demonstrated using a supramolecular assembly of fluorescein, which is a type II PS in the monomeric state; however, it changes to a type I SPS via self-assembly. The switching mechanism from type II to I via self-assembly was clarified using photophysical and electrochemical analyses, with the type I SPS exhibiting significant PDT effects on cancer cells. This study provides a promising approach for the development of type I PSs based on supramolecular assemblies.
@article{shigemitsu2022fluorescein, title = {Fluorescein-Based Type I Supramolecular Photosensitizer via Induction of Charge Separation by Self-Assembly}, author = {Shigemitsu, Hajime and Ohkubo, Kei and Sato, Kazuhide and Bunno, Asuka and Mori, Tadashi and Osakada, Yasuko and Fujitsuka, Mamoru and Kida, Toshiyuki}, journal = {JACS Au}, volume = {2}, issue = {6}, pages = {1472--1478}, year = {2022}, month = may, publisher = {ACS Publications}, doi = {10.1021/jacsau.2c00243}, url = {https://doi.org/10.1021/jacsau.2c00243}, dimensions = {true}, tab = {paper}, } -
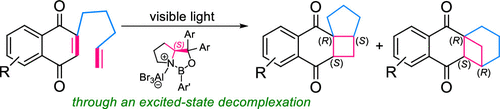 Visible Light-Induced Regio-and Enantiodifferentiating [2+2] Photocycloaddition of 1, 4-Naphthoquinones Mediated by Oppositely Coordinating 1, 3, 2-Oxazaborolidine Chiral Lewis AcidNao Shimizu, Hajime Shigemitsu, Toshiyuki Kida, Thorsten Bach, and Tadashi Mori*J. Org. Chem., 2022, 87, 8071–8083.
Visible Light-Induced Regio-and Enantiodifferentiating [2+2] Photocycloaddition of 1, 4-Naphthoquinones Mediated by Oppositely Coordinating 1, 3, 2-Oxazaborolidine Chiral Lewis AcidNao Shimizu, Hajime Shigemitsu, Toshiyuki Kida, Thorsten Bach, and Tadashi Mori*J. Org. Chem., 2022, 87, 8071–8083.A range of asymmetric photochemical transformations using visible light have recently become considerably attractive. Among the various approaches, chiral Lewis acid association to enones for [2 + 2] and ortho photocycloadditions and oxadi-π-methane rearrangements have shown to be very promising. Naturally, chiral Lewis acid coordination protects one of the prochiral faces of the C═C double bond, which enables an effective enantiodifferentiation in the following bond-forming process(es). Here, we studied regio- and enantiodifferentiating [2 + 2] photocycloaddition reactions of naphthoquinone derivatives mediated by chiral oxazaborolidines. A stereochemical control was quite challenging for the 2-ene-1,4-dione substrate, as a double coordination of Lewis acid essentially cancels out the face selectivity, and a mono-coordination to each carbonyl group leads to an opposite stereochemical outcome. Furthermore, a stepwise coordination in the ground state of Lewis acid in a 1:1 fashion was practically inaccessible. We found that the excited-state decomplexation is a key to accomplish high regio- and enantioselectivities in the photocycloaddition of an ene-dione.
@article{shimizu2022visible, title = {Visible Light-Induced Regio-and Enantiodifferentiating [2+2] Photocycloaddition of 1, 4-Naphthoquinones Mediated by Oppositely Coordinating 1, 3, 2-Oxazaborolidine Chiral Lewis Acid}, author = {Shimizu, Nao and Shigemitsu, Hajime and Kida, Toshiyuki and Bach, Thorsten and Mori, Tadashi}, journal = {J. Org. Chem.}, volume = {87}, issue = {12}, pages = {8071--8083}, year = {2022}, publisher = {ACS Publications}, doi = {10.1021/acs.joc.2c00730}, url = {https://doi.org/10.1021/acs.joc.2c00730}, dimensions = {true}, tab = {paper}, } -
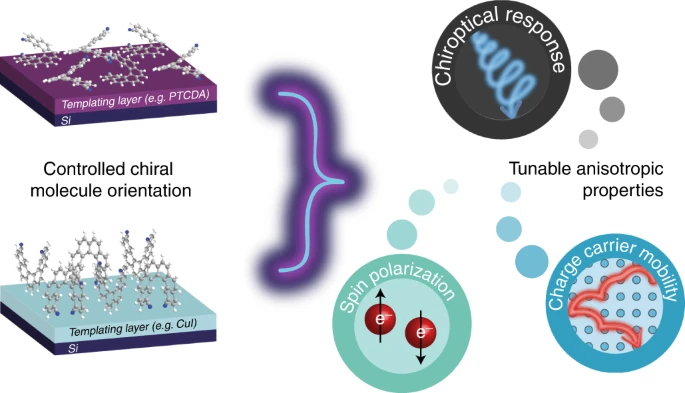 Controlling anisotropic properties by manipulating the orientation of chiral small moleculesJessica Wade*, Francesco Salerno, Rachel C Kilbride, Dong Kuk Kim, Julia A Schmidt, Joel A Smith, Luc M LeBlanc, Emma H Wolpert, Adebayo A Adeleke, Erin R Johnson, Jenny Nelson, Tadashi Mori, Kim E Jelfs, Sandrine Heutz, and Matthew J Fuchter*Nat. Chem., 2022, 14, 1383–1389.
Controlling anisotropic properties by manipulating the orientation of chiral small moleculesJessica Wade*, Francesco Salerno, Rachel C Kilbride, Dong Kuk Kim, Julia A Schmidt, Joel A Smith, Luc M LeBlanc, Emma H Wolpert, Adebayo A Adeleke, Erin R Johnson, Jenny Nelson, Tadashi Mori, Kim E Jelfs, Sandrine Heutz, and Matthew J Fuchter*Nat. Chem., 2022, 14, 1383–1389.Chiral π-conjugated molecules bring new functionality to technological applications and represent an exciting, rapidly expanding area of research. Their functional properties, such as the absorption and emission of circularly polarized light or the transport of spin-polarized electrons, are highly anisotropic. As a result, the orientation of chiral molecules critically determines the functionality and efficiency of chiral devices. Here we present a strategy to control the orientation of a small chiral molecule (2,2′-dicyano[6]helicene) by the use of organic and inorganic templating layers. Such templating layers can either force 2,2′-dicyano[6]helicene to adopt a face-on orientation and self-assemble into upright supramolecular columns oriented with their helical axis perpendicular to the substrate, or an edge-on orientation with parallel-lying supramolecular columns. Through such control, we show that low- and high-energy chiroptical responses can be independently ‘turned on’ or ‘turned off’. The templating methodologies described here provide a simple way to engineer orientational control and, by association, anisotropic functional properties of chiral molecular systems for a range of emerging technologies.
@article{wade2022controlling, title = {Controlling anisotropic properties by manipulating the orientation of chiral small molecules}, author = {Wade, Jessica and Salerno, Francesco and Kilbride, Rachel C and Kim, Dong Kuk and Schmidt, Julia A and Smith, Joel A and LeBlanc, Luc M and Wolpert, Emma H and Adeleke, Adebayo A and Johnson, Erin R and Nelson, Jenny and Mori, Tadashi and Jelfs, Kim E and Heutz, Sandrine and Fuchter, Matthew J}, journal = {Nat. Chem.}, volume = {14}, number = {12}, pages = {1383--1389}, year = {2022}, publisher = {Nature Publishing Group UK London}, doi = {10.1038/s41557-022-01044-6}, url = {https://doi.org/10.1038/s41557-022-01044-6}, dimensions = {true}, tab = {paper}, } -
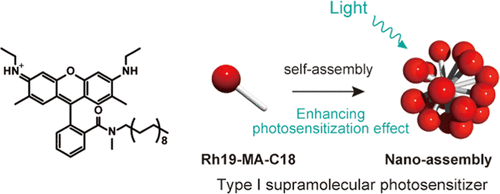 Amphiphilic rhodamine nano-assembly as a type I supramolecular photosensitizer for photodynamic therapyHajime Shigemitsu*, Kazuhide Sato, Satomi Hagio, Youhei Tani, Tadashi Mori, Kei Ohkubo, Yasuko Osakada, Mamoru Fujitsuka, and Toshiyuki Kida*ACS Appl. Nano Mater., 2022, 5, 14954–14960.
Amphiphilic rhodamine nano-assembly as a type I supramolecular photosensitizer for photodynamic therapyHajime Shigemitsu*, Kazuhide Sato, Satomi Hagio, Youhei Tani, Tadashi Mori, Kei Ohkubo, Yasuko Osakada, Mamoru Fujitsuka, and Toshiyuki Kida*ACS Appl. Nano Mater., 2022, 5, 14954–14960.Photodynamic therapy (PDT) is a promising clinical method for treating a wide range of cancers. Recently, PDT employing type I photosensitizers (PSs) has attracted considerable attention owing to the feasibility of efficient PDT under hypoxic conditions. Particularly, type I supramolecular PSs (SPSs) are promising candidates owing to their functional extensibility by facile hybridization. However, type I SPSs are rare, and the development strategy has not been established yet. In this work, we demonstrated that supramolecular assembly of a simple amphiphilic rhodamine 19 (Rh19-MA-C18) functioned as a type I SPS and exhibited significant PDT effect on cancer cells and cancer-bearing mice. This work demonstrates the potential of supramolecular nano-assembly composed of an amphiphilic rhodamine dye as an efficient type I SPS.
@article{shigemitsu2022amphiphilic, title = {Amphiphilic rhodamine nano-assembly as a type I supramolecular photosensitizer for photodynamic therapy}, author = {Shigemitsu, Hajime and Sato, Kazuhide and Hagio, Satomi and Tani, Youhei and Mori, Tadashi and Ohkubo, Kei and Osakada, Yasuko and Fujitsuka, Mamoru and Kida, Toshiyuki}, journal = {ACS Appl. Nano Mater.}, volume = {5}, number = {10}, pages = {14954--14960}, year = {2022}, publisher = {ACS Publications}, doi = {10.1021/acsanm.2c03192}, url = {https://doi.org/10.1021/acsanm.2c03192}, dimensions = {true}, tab = {paper}, } -
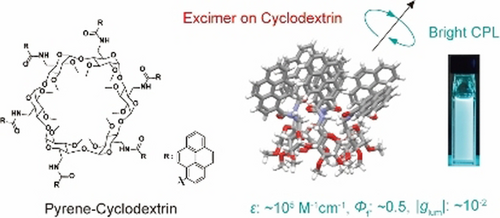 Cyclodextrins with multiple pyrenyl groups: an approach to organic molecules exhibiting bright excimer circularly polarized luminescenceHajime Shigemitsu*, Kosei Kawakami, Yuuya Nagata, Rikuo Kajiwara, Shintaro Yamada, Tadashi Mori, and Toshiyuki Kida*Angew. Chem. Int. Ed., 2022, 134, e202114700.
Cyclodextrins with multiple pyrenyl groups: an approach to organic molecules exhibiting bright excimer circularly polarized luminescenceHajime Shigemitsu*, Kosei Kawakami, Yuuya Nagata, Rikuo Kajiwara, Shintaro Yamada, Tadashi Mori, and Toshiyuki Kida*Angew. Chem. Int. Ed., 2022, 134, e202114700.We report a simple and effective approach to organic molecules exhibiting bright circularly polarized luminescence (CPL) by combining a chiral cyclic molecular scaffold and multiple excimer-enabling moieties. An α-cyclodextrin (CyD) scaffold was modified with six pyrenyl groups to obtain pyrene–cyclodextrins (PCDs) in a one-step synthesis from commercially available compounds. The PCDs exhibited high molar extinction coefficients (ϵ≈105 M−1 cm−1), polarized emission with a good dissymmetry factor (|glum|≈10−2), and quantum yield (Φf≈0.5). Owing to the excellent photophysical properties of the PCDs, the circularly polarized luminescence brightness (BCPL) reached 340 M−1 cm−1. Photophysical and chiroptical studies of the PCDs with only five pyrene units and with linkers of various lengths connecting the CyD with the pyrene units revealed that the formation of a pyrene excimer in a spatially crowded environment is crucial for CPL anisotropy. This study paves the way for the development of bright CPL organic molecules.
@article{shigemitsu2022cyclodextrins, title = {Cyclodextrins with multiple pyrenyl groups: an approach to organic molecules exhibiting bright excimer circularly polarized luminescence}, author = {Shigemitsu, Hajime and Kawakami, Kosei and Nagata, Yuuya and Kajiwara, Rikuo and Yamada, Shintaro and Mori, Tadashi and Kida, Toshiyuki}, journal = {Angew. Chem. Int. Ed.}, volume = {134}, number = {8}, pages = {e202114700}, year = {2022}, publisher = {Wiley Online Library}, doi = {10.1002/anie.202114700}, url = {https://doi.org/10.1002/anie.202114700}, dimensions = {true}, tab = {paper}, } -
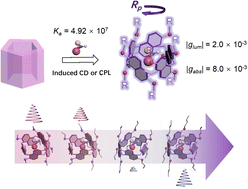 Chiroptical induction with prism [5] arene alkoxy-homologsXiaotong Liang, Yanling Shen, Dayang Zhou, Jiecheng Ji, Hongtao Wang, Ting Zhao, Tadashi Mori, Wanhua Wu, and Cheng YangChem. Commun., 2022, 58, 13584–13587.
Chiroptical induction with prism [5] arene alkoxy-homologsXiaotong Liang, Yanling Shen, Dayang Zhou, Jiecheng Ji, Hongtao Wang, Ting Zhao, Tadashi Mori, Wanhua Wu, and Cheng YangChem. Commun., 2022, 58, 13584–13587.The complexation of prism[5]arenes with amino acid derivatives showed association constants of up to 107 M−1, significant CD with gabs of up to 0.8 × 10−2 and CPL with glum of 2 × 10−3. The absolute configuration-CD signal correlation was established. The CD spectra varied significantly with the substituents on the prism[5]arenes.
@article{liang2022chiroptical, title = {Chiroptical induction with prism [5] arene alkoxy-homologs}, author = {Liang, Xiaotong and Shen, Yanling and Zhou, Dayang and Ji, Jiecheng and Wang, Hongtao and Zhao, Ting and Mori, Tadashi and Wu, Wanhua and Yang, Cheng}, journal = {Chem. Commun.}, volume = {58}, number = {98}, pages = {13584--13587}, year = {2022}, publisher = {Royal Society of Chemistry}, doi = {10.1039/d2cc05690a}, url = {https://doi.org/10.1039/d2cc05690a}, dimensions = {true}, tab = {paper}, } -
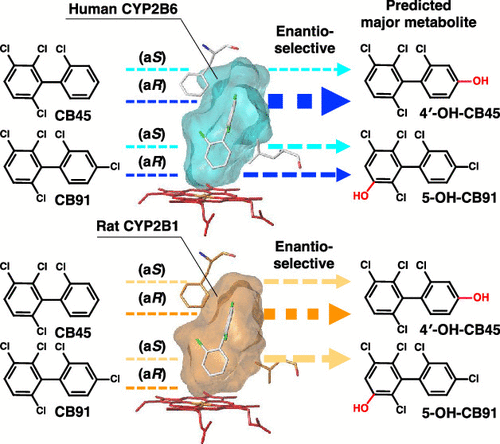 Differences in enantioselective hydroxylation of 2, 2’, 3, 6-tetrachlorobiphenyl (CB45) and 2, 2’, 3, 4’, 6-pentachlorobiphenyl (CB91) by human and rat CYP2B subfamiliesHideyuki Inui*, Terushi Ito, Chiharu Miwa, Yuki Haga, Makoto Kubo, Toshimasa Itoh, Keiko Yamamoto, Masayuki Miyaoka, Tadashi Mori, Harunobu Tsuzuki, and othersEnviron. Sci. Technol., 2022, 56, 10204–10215.
Differences in enantioselective hydroxylation of 2, 2’, 3, 6-tetrachlorobiphenyl (CB45) and 2, 2’, 3, 4’, 6-pentachlorobiphenyl (CB91) by human and rat CYP2B subfamiliesHideyuki Inui*, Terushi Ito, Chiharu Miwa, Yuki Haga, Makoto Kubo, Toshimasa Itoh, Keiko Yamamoto, Masayuki Miyaoka, Tadashi Mori, Harunobu Tsuzuki, and othersEnviron. Sci. Technol., 2022, 56, 10204–10215.Although polychlorinated biphenyls (PCBs) were commercially banned half a century ago, contamination of the environment and organisms by PCBs is still observed. PCBs show high persistence and bioaccumulation, resulting in toxicity. Among PCBs, chiral PCBs with more than three chlorine atoms at the ortho-position exhibit developmental and neurodevelopmental toxicity. Because toxicity is dependent on the atropisomer, atropisomer-specific metabolism is vital in determining toxicity. However, structural information on enantioselective metabolism remains elusive. Cytochrome P450 (CYP, P450) monooxygenases, particularly human CYP2B6 and rat CYP2B1, metabolize separated atropisomers of 2,2’,3,6-tetrachlorobiphenyl (CB45) and 2,2’,3,4’,6-pentachlorobiphenyl (CB91) to dechlorinated and hydroxylated metabolites. Docking studies using human CYP2B6 predict 4′-hydroxy (OH)-CB45 from (aR)-CB45 as a major metabolite of CB45. Di-OH- and dechlorinated OH-metabolites from human CYP2B6 and rat CYP2B1 are also detected. Several hydroxylated metabolites are derived from CB91 by both P450s; 5-OH-CB91 is predicted as a major metabolite. CB91 dechlorination is also detected by identifying 3-OH-CB51. A stable conformation of PCBs in the substrate-binding cavity and close distance to P450 heme are responsible for high metabolizing activities. As hydroxylation and dechlorination change PCB toxicity, this approach helps understand the possible toxicity of chiral PCBs in mammals.
@article{inui2022differences, title = {Differences in enantioselective hydroxylation of 2, 2', 3, 6-tetrachlorobiphenyl (CB45) and 2, 2', 3, 4', 6-pentachlorobiphenyl (CB91) by human and rat CYP2B subfamilies}, author = {Inui, Hideyuki and Ito, Terushi and Miwa, Chiharu and Haga, Yuki and Kubo, Makoto and Itoh, Toshimasa and Yamamoto, Keiko and Miyaoka, Masayuki and Mori, Tadashi and Tsuzuki, Harunobu and others}, journal = {Environ. Sci. Technol.}, volume = {56}, number = {14}, pages = {10204--10215}, year = {2022}, publisher = {ACS Publications}, doi = {10.1021/acs.est.2c01155}, url = {https://doi.org/10.1021/acs.est.2c01155}, dimensions = {true}, tab = {paper}, } - Photochemistry Controlled by Weak Supramolecular InteractionsTadashi Mori*Manufacturing and Technology, 2022, 74, 65–68.
@article{mori2022photochemistry, title = {Photochemistry Controlled by Weak Supramolecular Interactions}, author = {Mori, Tadashi}, journal = {Manufacturing and Technology}, volume = {74}, issue = {1}, pages = {65--68}, year = {2022} }