articles in 2021
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
 Overtemperature-protection intelligent molecular chiroptical photoswitchesJiabin Yao, Wanhua Wu*, Chao Xiao, Dan Su, Zhihui Zhong, Tadashi Mori, and Cheng Yang*Nat. Commun., 2021, 12, 2600.
Overtemperature-protection intelligent molecular chiroptical photoswitchesJiabin Yao, Wanhua Wu*, Chao Xiao, Dan Su, Zhihui Zhong, Tadashi Mori, and Cheng Yang*Nat. Commun., 2021, 12, 2600.Stimuli-responsive intelligent molecular machines/devices are of current research interest due to their potential application in minimized devices. Constructing molecular machines/devices capable of accomplishing complex missions is challenging, demanding coalescence of various functions into one molecule. Here we report the construction of intelligent molecular chiroptical photoswitches based on azobenzene-fused bicyclic pillar[n]arene derivatives, which we defined as molecular universal joints (MUJs). The Z/E photoisomerization of the azobenzene moiety of MUJs induces rolling in/out conformational switching of the azobenzene-bearing side-ring and consequently leads to planar chirality switching of MUJs. Meanwhile, temperature variation was demonstrated to also cause conformational/chiroptical inversion due to the significant entropy change during the ring-flipping. As a result, photo-induced chiroptical switching could be prohibited when the temperature exceeded an upper limit, demonstrating an intelligent molecular photoswitch having over-temperature protection function, which is in stark contrast to the low-temperature-gating effect commonly encountered.
@article{yao2021overtemperature, title = {Overtemperature-protection intelligent molecular chiroptical photoswitches}, author = {Yao, Jiabin and Wu, Wanhua and Xiao, Chao and Su, Dan and Zhong, Zhihui and Mori, Tadashi and Yang, Cheng}, journal = {Nat. Commun.}, volume = {12}, issue = {1}, pages = {2600}, year = {2021}, publisher = {Nature Publishing Group UK London}, doi = {10.1038/s41467-021-22880-z}, url = {https://doi.org/10.1038/s41467-021-22880-z}, dimensions = {true}, tab = {paper}, } -
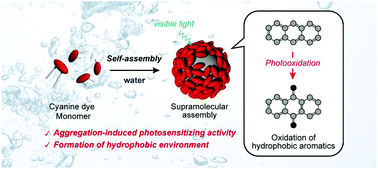 A cyanine dye based supramolecular photosensitizer enabling visible-light-driven organic reaction in waterHajime Shigemitsu*, Tomoe Tamemoto, Kei Ohkubo, Tadashi Mori, Yasuko Osakada, Mamoru Fujitsuka, and Toshiyuki KidaChem. Commun., 2021, 57, 11217–11220.
A cyanine dye based supramolecular photosensitizer enabling visible-light-driven organic reaction in waterHajime Shigemitsu*, Tomoe Tamemoto, Kei Ohkubo, Tadashi Mori, Yasuko Osakada, Mamoru Fujitsuka, and Toshiyuki KidaChem. Commun., 2021, 57, 11217–11220.We report the aggregation-induced photosensitizing activity of a cyanine dye in water and the mechanism. In addition, using the supramolecular assembly, visible-light-driven photooxidation of hydrophobic aromatic compounds in water was successfully performed.
@article{shigemitsu2021cyanine, title = {A cyanine dye based supramolecular photosensitizer enabling visible-light-driven organic reaction in water}, author = {Shigemitsu, Hajime and Tamemoto, Tomoe and Ohkubo, Kei and Mori, Tadashi and Osakada, Yasuko and Fujitsuka, Mamoru and Kida, Toshiyuki}, journal = {Chem. Commun.}, volume = {57}, issue = {85}, pages = {11217--11220}, year = {2021}, publisher = {Royal Society of Chemistry}, doi = {10.1039/d1cc04685c}, url = {https://doi.org/10.1039/d1cc04685c}, dimensions = {true}, tab = {paper}, } -
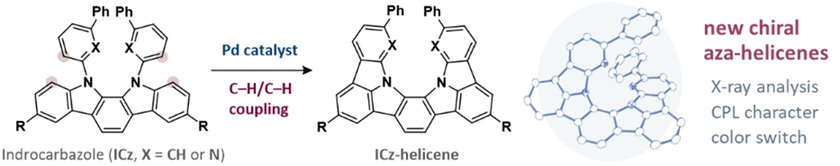 Synthesis, Structure, and Chiroptical Properties of Indolo-and Pyridopyrrolo-Carbazole-Based C2-Symmetric AzahelicenesTaisei Taniguchi, Yuji Nishii, Tadashi Mori*, Ken-ichi Nakayama, and Masahiro Miura*Chem. Eur. J., 2021, 27, 7356–7361.
Synthesis, Structure, and Chiroptical Properties of Indolo-and Pyridopyrrolo-Carbazole-Based C2-Symmetric AzahelicenesTaisei Taniguchi, Yuji Nishii, Tadashi Mori*, Ken-ichi Nakayama, and Masahiro Miura*Chem. Eur. J., 2021, 27, 7356–7361.Treatment of 11,12-bis(1,1’-biphenyl-3-yl or 6-phenylpyridin-2-yl)-substituted 11,12-dihydro-indolo[2,3-a]carbazole with an oxidizing system of Pd(II)/Ag(I) induced effective double dehydrogenative cyclization to afford the corresponding π-extended azahelicenes. The optical resolutions were readily achieved by a preparative chiral HPLC. It was found that the pyridopyrrolo-carbazole-based azahelicene that contains four nitrogen atoms exhibits ca. 6 times larger dissymmetry factors both in circularly dichroism (CD) and circularly polarized luminescence (CPL), |gCD| and |gCPL| values being 1.1×10−2 and 4.4×10−3, respectively, as compared with the parent indolocarbazole-based azahelicene. Theoretical calculations at the RI-CC2 level were employed to rationalize the observed enhanced chiroptical responses. The (chir)optical properties of the former helicene was further tuned by a protonation leading to remarkable red-shift with a considerable enhancement of the |gCPL| value.
@article{taniguchi2021synthesis, title = {Synthesis, Structure, and Chiroptical Properties of Indolo-and Pyridopyrrolo-Carbazole-Based C2-Symmetric Azahelicenes}, author = {Taniguchi, Taisei and Nishii, Yuji and Mori, Tadashi and Nakayama, Ken-ichi and Miura, Masahiro}, journal = {Chem. Eur. J.}, volume = {27}, issue = {26}, pages = {7356--7361}, year = {2021}, publisher = {Wiley Online Library}, doi = {10.1002/chem.202100327}, url = {https://doi.org/10.1002/chem.202100327}, dimensions = {true}, tab = {paper}, } -
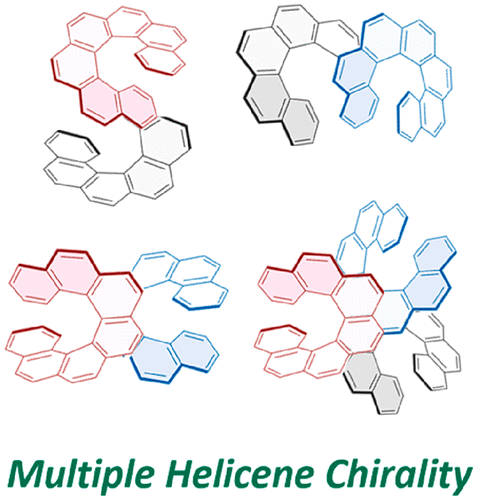 Chiroptical properties of symmetric double, triple, and multiple helicenesTadashi Mori*Chem. Rev., 2021, 121, 2373–2412.
Chiroptical properties of symmetric double, triple, and multiple helicenesTadashi Mori*Chem. Rev., 2021, 121, 2373–2412.Helicenes have attracted considerable attention due to their inherent helical chirality and extended π-conjugation. Recently, rapid progress has been witnessed in the preparation of double, triple, quadruple, quintuple, and sextuple helicenes, where plural helicene moieties are symmetrically arranged in a single molecule. While synthetic efforts and X-ray crystallographic analyses devoted to these multiple helicenes and theoretical investigations on their isomerization and racemization behaviors have been relatively well documented and reviewed in the literature, the chiroptical properties of the multiple helicities have been somewhat overlooked. This review discourses the cumulative and systematic investigations on the chiroptical properties such as the circular dichroism (CD) and circularly polarized luminescence (CPL) of multiple helicenes. Although the number and structural variations of multiple helicenes reported to date have been fairly limited, this review overviews the current status of the chemistry of multiple helicenes from the viewpoint of chiroptical properties and provides insights into the design principle for advanced chiroptical materials through the proper arrangement of multiple helices, highlighting the impact of the molecular symmetry on the chiroptical responses.
@article{mori2021chiroptical, title = {Chiroptical properties of symmetric double, triple, and multiple helicenes}, author = {Mori, Tadashi}, journal = {Chem. Rev.}, volume = {121}, issue = {4}, pages = {2373--2412}, year = {2021}, publisher = {ACS Publications}, doi = {10.1021/acs.chemrev.0c01017}, url = {https://doi.org/10.1021/acs.chemrev.0c01017}, dimensions = {true}, tab = {review}, }