articles in 2019
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
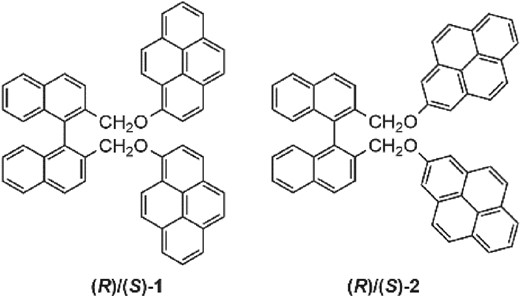 Sign control of circularly polarized luminescence based on geometric arrangement of fluorescent pyrene units in a binaphthyl scaffoldDaiki Kaji, Shintaro Ikeda, Kenya Takamura, Nobuo Tajima, Motohiro Shizuma, Tadashi Mori, Makoto Miyasaka*, and Yoshitane Imai*Chem. Lett., 2019, 48, 874–876.
Sign control of circularly polarized luminescence based on geometric arrangement of fluorescent pyrene units in a binaphthyl scaffoldDaiki Kaji, Shintaro Ikeda, Kenya Takamura, Nobuo Tajima, Motohiro Shizuma, Tadashi Mori, Makoto Miyasaka*, and Yoshitane Imai*Chem. Lett., 2019, 48, 874–876.The signs of circularly polarized luminescence and circular dichroism of binaphthyl-pyrene fluorophores with the same axial chirality can be controlled by altering the bonding positions of the two fluorescent pyrene units in the solution state. The relative geometrical arrangement of the two pyrene rings plays a substantial role in determining the signs of the observed chiroptical properties.
@article{kaji2019sign, title = {Sign control of circularly polarized luminescence based on geometric arrangement of fluorescent pyrene units in a binaphthyl scaffold}, author = {Kaji, Daiki and Ikeda, Shintaro and Takamura, Kenya and Tajima, Nobuo and Shizuma, Motohiro and Mori, Tadashi and Miyasaka, Makoto and Imai, Yoshitane}, journal = {Chem. Lett.}, volume = {48}, issue = {8}, pages = {874--876}, year = {2019}, publisher = {Oxford University Press}, doi = {10.1246/cl.190246}, url = {https://doi.org/10.1246/cl.190246}, dimensions = {true}, tab = {paper}, } -
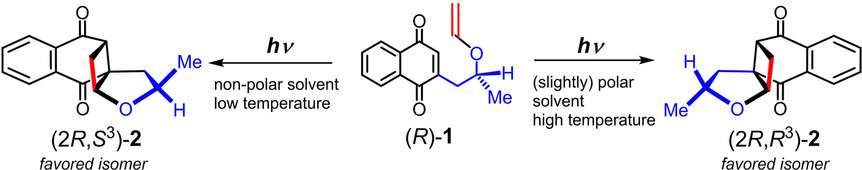 Diastereoselective Photocycloaddition Reaction of Vinyl Ether Tethered to 1, 4-NaphthoquinoneHiroki Ishikawa, Tim S Chung, Gaku Fukuhara, Hajime Shigemitsu, Toshiyuki Kida, Thorsten Bach, and Tadashi Mori*ChemPhotoChem, 2019, 3, 243–250.
Diastereoselective Photocycloaddition Reaction of Vinyl Ether Tethered to 1, 4-NaphthoquinoneHiroki Ishikawa, Tim S Chung, Gaku Fukuhara, Hajime Shigemitsu, Toshiyuki Kida, Thorsten Bach, and Tadashi Mori*ChemPhotoChem, 2019, 3, 243–250.The intramolecular asymmetric photocycloaddition between 1,4-naphthoquinone and vinyl ether with a small (R)-point-chiral group on the tether was studied. Under photoirradiation, [2+2] cyclobutane products were exclusively obtained for this intramolecular system. The effect of solvent polarity on the stereoselectivity was significant, with predominant formation of the (2R,S3) over the (2R,R3) isomer in non-polar solvents being reversed in slightly polar dichloromethane. The detailed temperature-dependent study revealed the dominant diastereo-differentiating processes, which were switched between the ground-state equilibrium, the relative rate of bond formation in the triplet manifold, as well as deactivation processes between the pro-(S3) and pro-(R3) precursors, depending on the temperature domain examined. The enthalpic contribution (ΔΔH≠) was always compensated by the entropic factor (ΔΔS≠), implying the importance of solvation on the diastereo-differentiation steps. The mechanism of photocycloaddition, especially for the face-selective processes, is thoroughly discussed, which is supported by quantum chemical calculations on the ground-state circular dichroism (CD) spectral behavior as well as on the diastereomeric transition states in the triplet excited state.
@article{ishikawa2019diastereoselective, title = {Diastereoselective Photocycloaddition Reaction of Vinyl Ether Tethered to 1, 4-Naphthoquinone}, author = {Ishikawa, Hiroki and Chung, Tim S and Fukuhara, Gaku and Shigemitsu, Hajime and Kida, Toshiyuki and Bach, Thorsten and Mori, Tadashi}, journal = {ChemPhotoChem}, volume = {3}, issue = {5}, pages = {243--250}, year = {2019}, publisher = {Wiley Online Library}, doi = {10.1002/cptc.201900022}, url = {https://doi.org/10.1002/cptc.201900022}, dimensions = {true}, tab = {paper}, } -
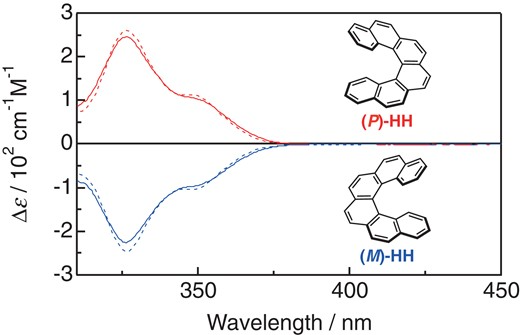 Transient circular dichroism measurement of the excited triplet state of pristine hexahelicene in solution at room temperatureMakoto Kuronuma, Takehito Sato, Yasuyuki Araki*, Tadashi Mori, Seiji Sakamoto, Yoshihisa Inoue, Osamu Ito, and Takehiko Wada*Chem. Lett., 2019, 48, 357–360.
Transient circular dichroism measurement of the excited triplet state of pristine hexahelicene in solution at room temperatureMakoto Kuronuma, Takehito Sato, Yasuyuki Araki*, Tadashi Mori, Seiji Sakamoto, Yoshihisa Inoue, Osamu Ito, and Takehiko Wada*Chem. Lett., 2019, 48, 357–360.The intramolecular asymmetric photocycloaddition between 1,4-naphthoquinone and vinyl ether with a small (R)-point-chiral group on the tether was studied. Under photoirradiation, [2+2] cyclobutane products were exclusively obtained for this intramolecular system. The effect of solvent polarity on the stereoselectivity was significant, with predominant formation of the (2R,S3) over the (2R,R3) isomer in non-polar solvents being reversed in slightly polar dichloromethane. The detailed temperature-dependent study revealed the dominant diastereo-differentiating processes, which were switched between the ground-state equilibrium, the relative rate of bond formation in the triplet manifold, as well as deactivation processes between the pro-(S3) and pro-(R3) precursors, depending on the temperature domain examined. The enthalpic contribution (ΔΔH≠) was always compensated by the entropic factor (ΔΔS≠), implying the importance of solvation on the diastereo-differentiation steps. The mechanism of photocycloaddition, especially for the face-selective processes, is thoroughly discussed, which is supported by quantum chemical calculations on the ground-state circular dichroism (CD) spectral behavior as well as on the diastereomeric transition states in the triplet excited state.
@article{kuronuma2019transient, title = {Transient circular dichroism measurement of the excited triplet state of pristine hexahelicene in solution at room temperature}, author = {Kuronuma, Makoto and Sato, Takehito and Araki, Yasuyuki and Mori, Tadashi and Sakamoto, Seiji and Inoue, Yoshihisa and Ito, Osamu and Wada, Takehiko}, journal = {Chem. Lett.}, volume = {48}, issue = {4}, pages = {357--360}, year = {2019}, publisher = {Oxford University Press}, doi = {10.1246/cl.190012}, url = {https://doi.org/10.1246/cl.190012}, dimensions = {true}, tab = {paper}, } -
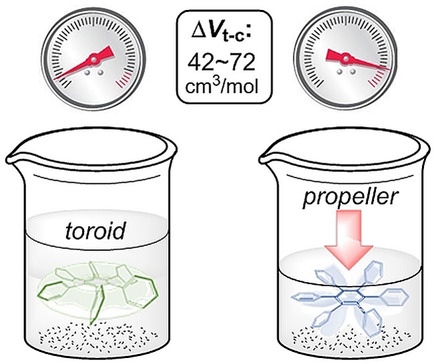 Hydrostatic pressure on toroidal interaction and propeller chirality of hexaarylbenzenes: explicit solvent effects on differential volumes in methylcyclohexane and hexaneTomoyo Kosaka, Satono Iwai, Gaku Fukuhara*, Yoshitane Imai, and Tadashi Mori*Chem. Eur. J., 2019, 25, 2011–2018.
Hydrostatic pressure on toroidal interaction and propeller chirality of hexaarylbenzenes: explicit solvent effects on differential volumes in methylcyclohexane and hexaneTomoyo Kosaka, Satono Iwai, Gaku Fukuhara*, Yoshitane Imai, and Tadashi Mori*Chem. Eur. J., 2019, 25, 2011–2018.A unique and effective interaction between the peripheral aromatic blades makes hexaarylbenzenes (HABs) attractive in fundamental research as well as for various applications such as molecular wires, sensors, and supramolecular assemblies. The chiroptical responses of HABs are susceptible to environmental factors such as solvent and temperature owing to the dynamic conformational transitions between the conformers. In this study, pressure dependence on the propeller chiral HABs in two different solvents was studied in detail. The effective differential volumes for two different equilibria were determined by quantitative analyses of CD spectra, affording very large differential volumes from the propeller to toroidal conformer (ΔVT-C) of +43 and +42 cm3 mol−1, for H2 and H6, respectively, in methylcyclohexane. The value of H6 was further enhanced to +72 cm3 mol−1 in hexane, the largest value for the typical unimolecular conformational change. Such a response of propeller chirality in HABs is expedient in designing more advanced piezo-sensitive materials.
@article{kosaka2019hydrostatic, title = {Hydrostatic pressure on toroidal interaction and propeller chirality of hexaarylbenzenes: explicit solvent effects on differential volumes in methylcyclohexane and hexane}, author = {Kosaka, Tomoyo and Iwai, Satono and Fukuhara, Gaku and Imai, Yoshitane and Mori, Tadashi}, journal = {Chem. Eur. J.}, volume = {25}, issue = {8}, pages = {2011--2018}, year = {2019}, publisher = {Wiley Online Library}, doi = {10.1002/chem.201804688}, url = {https://doi.org/10.1002/chem.201804688}, dimensions = {true}, tab = {paper}, } -
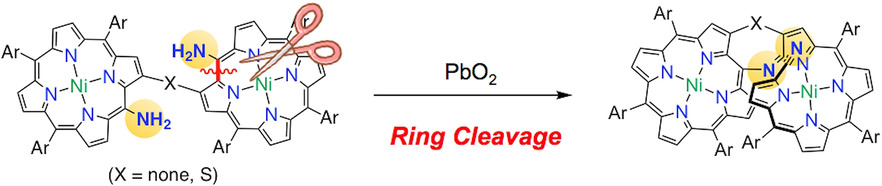 Selective Formation of Helical Tetrapyrrin-Fused Porphyrins by Oxidation of β-to-β Linked meso-Aminoporphyrin DimersKeisuke Fujimoto, Daiki Shimizu, Tadashi Mori, Yuanyuan Li, Mingbo Zhou, Jianxin Song, and Atsuhiro Osuka*Chem. Eur. J., 2019, 25, 1711–1715.
Selective Formation of Helical Tetrapyrrin-Fused Porphyrins by Oxidation of β-to-β Linked meso-Aminoporphyrin DimersKeisuke Fujimoto, Daiki Shimizu, Tadashi Mori, Yuanyuan Li, Mingbo Zhou, Jianxin Song, and Atsuhiro Osuka*Chem. Eur. J., 2019, 25, 1711–1715.Oxidation of β-to-β directly linked and sulfur-bridged meso-amino NiII-porphyrin dimers with PbO2 gave helical tetrapyrrin (biliverdin analogue)-fused NiII-porphyrins. These ring cleaving reactions differ markedly from the previously reported oxidation of a β–β linked NiII-porphyrin dimer carrying one amino group, which gave an azepine-fused porphyrin dimer. The tetrapyrrin-fused NiII-porphyrins display intense NIR absorption bands at 1200–1400 nm and reversible redox processes because of the highly π-conjugated networks and rigid structures. These tetrapyrrin-fused NiII-porphyrins were separated to stable enantiomers, which showed clear Cotton effects in their CD spectra with Δϵ of 102 order.
@article{fujimoto2019selective, title = {Selective Formation of Helical Tetrapyrrin-Fused Porphyrins by Oxidation of β-to-β Linked meso-Aminoporphyrin Dimers}, author = {Fujimoto, Keisuke and Shimizu, Daiki and Mori, Tadashi and Li, Yuanyuan and Zhou, Mingbo and Song, Jianxin and Osuka, Atsuhiro}, journal = {Chem. Eur. J.}, volume = {25}, issue = {7}, pages = {1711--1715}, year = {2019}, publisher = {Wiley Online Library}, doi = {10.1002/chem.201805659}, url = {https://doi.org/10.1002/chem.201805659}, dimensions = {true}, tab = {paper}, } -
 An ultimate stereocontrol in supramolecular photochirogenesis: photocyclodimerization of 2-anthracenecarboxylate mediated by sulfur-linked β-cyclodextrin dimersJiecheng Ji, Wanhua Wu, Wenting Liang, Guo Cheng, Ryohei Matsushita, Zhiqiang Yan, Xueqin Wei, Ming Rao, De-Qi Yuan, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cheng Yang*J. Am. Chem. Soc., 2019, 141, 9225–9238.
An ultimate stereocontrol in supramolecular photochirogenesis: photocyclodimerization of 2-anthracenecarboxylate mediated by sulfur-linked β-cyclodextrin dimersJiecheng Ji, Wanhua Wu, Wenting Liang, Guo Cheng, Ryohei Matsushita, Zhiqiang Yan, Xueqin Wei, Ming Rao, De-Qi Yuan, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cheng Yang*J. Am. Chem. Soc., 2019, 141, 9225–9238.Stereoisomeric β-cyclodextrin (CD) dimers linked with a sulfur atom or an arene spacer were designed to create a tethered dual CD capsule for precisely manipulating the regio- and enantioselectivities of the photocyclodimerization of 2-anthracenecarboxylate (AC) to four stereoisomeric classical 9,10:9′,10′-cyclodimers and two nonclassical 5,8:9′,10′-cyclodimers. Among the dimeric CD hosts prepared, exo-3-thia-β-CD dimer formed 1:1 and 1:2 host–guest complexes with AC in aqueous solutions, the former of which hindered but the latter facilitated the AC photocyclodimerization with regio- and enantioselectivities much higher than those obtained with native β-CD or the rest of the β-CD dimers. The stereochemical outcomes turned out to be highly sensitive to and hence critically manipulable by the linking position and configuration of the connected saccharide units and the linker length, as well as the external variants, such as temperature, pH, and added salt. Eventually, the photocyclodimerization of AC mediated by the dimeric β-CD host gave enantiopure syn-head-to-tail-9,10:9′,10′-cyclodimer in 97–98% yield in a pH 5.1 buffer solution at 0.5 °C and also in an aqueous CsCl solution at −20 °C.
@article{ji2019ultimate, title = {An ultimate stereocontrol in supramolecular photochirogenesis: photocyclodimerization of 2-anthracenecarboxylate mediated by sulfur-linked β-cyclodextrin dimers}, author = {Ji, Jiecheng and Wu, Wanhua and Liang, Wenting and Cheng, Guo and Matsushita, Ryohei and Yan, Zhiqiang and Wei, Xueqin and Rao, Ming and Yuan, De-Qi and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa and Yang, Cheng}, journal = {J. Am. Chem. Soc.}, volume = {141}, number = {23}, pages = {9225--9238}, year = {2019}, publisher = {ACS Publications}, doi = {10.1021/jacs.9b01993}, url = {https://doi.org/10.1021/jacs.9b01993}, dimensions = {true}, tab = {paper}, }