articles in 2018
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
 Spiroborate-based double-stranded helicates: meso-to-racemo isomerization and ion-triggered springlike motion of the racemo-helicateNaoki Ousaka, Kaori Shimizu, Yoshimasa Suzuki, Takuya Iwata, Manabu Itakura, Daisuke Taura, Hiroki Iida, Yoshio Furusho, Tadashi Mori, and Eiji Yashima*J. Am. Chem. Soc., 2018, 140, 17027–17039.
Spiroborate-based double-stranded helicates: meso-to-racemo isomerization and ion-triggered springlike motion of the racemo-helicateNaoki Ousaka, Kaori Shimizu, Yoshimasa Suzuki, Takuya Iwata, Manabu Itakura, Daisuke Taura, Hiroki Iida, Yoshio Furusho, Tadashi Mori, and Eiji Yashima*J. Am. Chem. Soc., 2018, 140, 17027–17039.A one-handed double-stranded spiroborate helicate exhibits a unique reversible extension–contraction motion coupled with a twisting motion in one direction triggered by binding and release of a Na+ ion while retaining its handedness. Here we report that an extended meso-helicate was also produced together with the racemo-helicate, and the meso-helicate was readily converted to the racemo-helicate assisted by a Na+ ion as a template in the presence of water. The thermodynamic analyses of the ion-triggered springlike motion of the racemo-helicate using a series of monovalent cations with different sizes revealed that the association constants of the extended racemo-helicate decreased in the following order: Li+ > Na+ > NH4+ > Ag+ ≥ K+ > Cs+ > Rb+, which roughly agrees with the reverse order of their ionic radii except for the NH4+ ion due to the more elongated contracted helicates when complexed with larger cations as supported by the crystal and DFT calculated structures. The one-handed contracted helicates showed characteristic CD spectra depending on the cation species due to the differences in their contracted helical structures, and its absolute handedness of the spiroborate helicate was determined by X-ray crystallography. The kinetic studies of the springlike motions of the racemo-helicate showed that the exchange rate between the extended and contracted helicates tend to increase in the following order: Li+ < Na+ < K+ ≤ NH4+ < Rb+ < Cs+ < Ag+ as anticipated from the association constants, being in good agreement with the order of the cation sizes except for Ag+.
@article{ousaka2018spiroborate, title = {Spiroborate-based double-stranded helicates: meso-to-racemo isomerization and ion-triggered springlike motion of the racemo-helicate}, author = {Ousaka, Naoki and Shimizu, Kaori and Suzuki, Yoshimasa and Iwata, Takuya and Itakura, Manabu and Taura, Daisuke and Iida, Hiroki and Furusho, Yoshio and Mori, Tadashi and Yashima, Eiji}, journal = {J. Am. Chem. Soc.}, volume = {140}, issue = {49}, pages = {17027--17039}, year = {2018}, publisher = {ACS Publications}, doi = {10.1021/jacs.8b08268}, url = {https://doi.org/10.1021/jacs.8b08268}, dimensions = {true}, tab = {paper}, } -
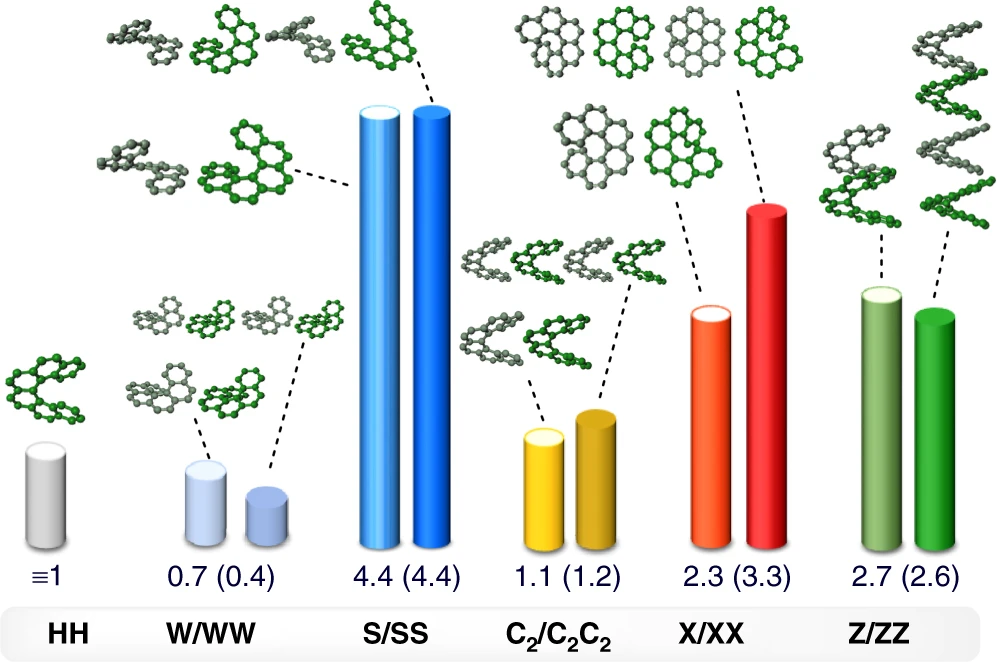 Symmetry-based rational design for boosting chiroptical responsesHiroki Tanaka, Mina Ikenosako, Yuka Kato, Michiya Fujiki, Yoshihisa Inoue, and Tadashi Mori*Communications Chemistry, 2018, 1, 38.
Symmetry-based rational design for boosting chiroptical responsesHiroki Tanaka, Mina Ikenosako, Yuka Kato, Michiya Fujiki, Yoshihisa Inoue, and Tadashi Mori*Communications Chemistry, 2018, 1, 38.Chiral molecules play indispensable roles in advanced materials and technologies. Nevertheless, no conventional, yet reliable logical strategies are available for designing chiral molecules of desired chiroptical properties. Here, we propose a general protocol for rationally aligning multiple chiral units to boost the chiroptical responses, using hexahelicene as a prototype. In this proof-of-concept study, we align two hexahelicenes in various orientations and examine by theoretical calculations to predict the best chiroptical performance for X-shaped and S-shaped double hexahelicenes. We synthesize and optically resolve both double hexahelicenes and show that they exhibit more than a twofold increase in intensity of circular dichroism and circularly polarized luminescence, experimentally validating the protocol. The enhanced chiroptical responses are theoretically assignable to the electric and magnetic transition dipole moments of component hexahelicenes aligned in the correct symmetry. A guiding principle for designing advanced molecular and supramolecular chiral materials is further discussed.
@article{tanaka2018symmetry, title = {Symmetry-based rational design for boosting chiroptical responses}, author = {Tanaka, Hiroki and Ikenosako, Mina and Kato, Yuka and Fujiki, Michiya and Inoue, Yoshihisa and Mori, Tadashi}, journal = {Communications Chemistry}, volume = {1}, issue = {1}, pages = {38}, year = {2018}, publisher = {Nature Publishing Group UK London}, doi = {10.1038/s42004-018-0035-x}, url = {https://doi.org/10.1038/s42004-018-0035-x}, dimensions = {true}, tab = {paper}, } -
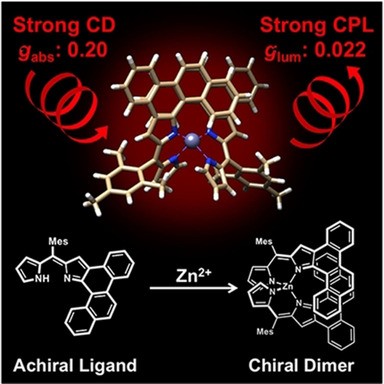 Significant Enhancement of Absorption and Luminescence Dissymmetry Factors in the Far-Red Region: A Zinc (II) Homoleptic Helicate Formed by a Pair of Achiral Dipyrromethene LigandsHiroaki Ito, Hayato Sakai, Yoshinori Okayasu, Junpei Yuasa, Tadashi Mori*, and Taku Hasobe*Chem. Eur. J., 2018, 24, 16889–16894.
Significant Enhancement of Absorption and Luminescence Dissymmetry Factors in the Far-Red Region: A Zinc (II) Homoleptic Helicate Formed by a Pair of Achiral Dipyrromethene LigandsHiroaki Ito, Hayato Sakai, Yoshinori Okayasu, Junpei Yuasa, Tadashi Mori*, and Taku Hasobe*Chem. Eur. J., 2018, 24, 16889–16894.A homoleptic zinc(II) helicate organized by a pair of achiral dipyrromethene ligands through zinc(II) coordination was synthesized to evaluate the chiroptical properties. This zinc complex showed strong exciton-coupled chiroptical responses from the helical configuration with a large absorption dissymmetry factor |gabs| (up to 0.20). More importantly, intense polarized luminescence in the far-red region (700–850 nm) with a fluorescence quantum yield ΦFL of 0.23 was observed for this helicate with a dissymmetry factor |glum| of 0.022, the largest value among rare-earth- and precious-metal-free small molecules. These unprecedentedly large g values were supported by theoretical calculations.
@article{ito2018significant, title = {Significant Enhancement of Absorption and Luminescence Dissymmetry Factors in the Far-Red Region: A Zinc (II) Homoleptic Helicate Formed by a Pair of Achiral Dipyrromethene Ligands}, author = {Ito, Hiroaki and Sakai, Hayato and Okayasu, Yoshinori and Yuasa, Junpei and Mori, Tadashi and Hasobe, Taku}, journal = {Chem. Eur. J.}, volume = {24}, issue = {63}, pages = {16889--16894}, year = {2018}, publisher = {Wiley Online Library}, doi = {10.1002/chem.201804171}, url = {https://doi.org/10.1002/chem.201804171}, dimensions = {true}, tab = {paper}, } -
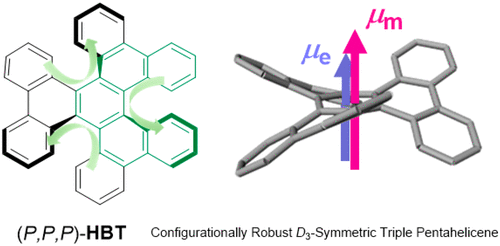 Combined Experimental and Theoretical Study on Circular Dichroism and Circularly Polarized Luminescence of Configurationally Robust D3-Symmetric Triple PentaheliceneHiroki Tanaka, Yuka Kato, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. A, 2018, 122, 7378–7384.
Combined Experimental and Theoretical Study on Circular Dichroism and Circularly Polarized Luminescence of Configurationally Robust D3-Symmetric Triple PentaheliceneHiroki Tanaka, Yuka Kato, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. A, 2018, 122, 7378–7384.Pentahelicene (PH) exhibits the largest absorption (gabs) and luminescence (glum) dissymmetry factors among the helicene family but is configurationally and (photo)chemically labile, encumbering its application to chiroptical materials. To bypass the pitfalls, three PH units are merged in a single molecule to build D3-symmetric triple pentahelicene, hexabenzotriphenylene (HBT), which attains indeed the configurational and (photo)chemical robustness through equilibrium with a C2-symmetric conformer that interrupts the racemization and photocyclization. UV–vis, circular dichroism (CD), and circularly polarized luminescence (CPL) spectral examinations reveal the significantly larger gabs and glum values for HBT than for any of configurationally robust single [n]helicene (n ≥ 6) and C2-symmetric triple pentahelicene, trinaphthotriphenylene (TNT). Theoretical calculations precisely reproduce the main features of the experimental CD and CPL spectra of PH, HBT, and TNT, and the relevant electric and magnetic transition moments and their mutual angles well rationalize the relative CD and CPL intensities of all the single and triple pentahelicenes.
@article{tanaka2018combined, title = {Combined Experimental and Theoretical Study on Circular Dichroism and Circularly Polarized Luminescence of Configurationally Robust D3-Symmetric Triple Pentahelicene}, author = {Tanaka, Hiroki and Kato, Yuka and Inoue, Yoshihisa and Mori, Tadashi}, journal = {J. Phys. Chem. A}, volume = {122}, issue = {37}, pages = {7378--7384}, year = {2018}, publisher = {ACS Publications}, doi = {10.1021/acs.jpca.8b05247}, url = {https://doi.org/10.1021/acs.jpca.8b05247}, dimensions = {true}, tab = {paper}, } -
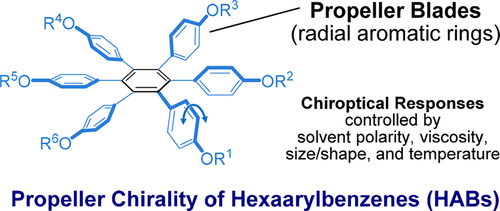 Solvent and temperature effects on dynamics and chiroptical properties of propeller chirality and toroidal interaction of hexaarylbenzenesTomoyo Kosaka, Satono Iwai, Yoshihisa Inoue, Toshiyuki Moriuchi, and Tadashi Mori*The Journal of Physical Chemistry A, 2018, 122, 7455–7463.
Solvent and temperature effects on dynamics and chiroptical properties of propeller chirality and toroidal interaction of hexaarylbenzenesTomoyo Kosaka, Satono Iwai, Yoshihisa Inoue, Toshiyuki Moriuchi, and Tadashi Mori*The Journal of Physical Chemistry A, 2018, 122, 7455–7463.Because of the unique interaction of radial aromatic blades, propeller-shaped hexaarylbenzenes (HABs) attract much research interest and find various practical applications. By introducing a small point-chiral group at the tip of aromatic blade(s), HAB becomes propeller-chiral to exhibit strong Cotton effects. Because of the dynamic nature of propeller chirality, the chiroptical properties of HAB critically responded to minute changes in the environment. Using a series of chiral HABs with one to six (R)-1-methylpropyloxy substituent(s) introduced at the blade tip, we elucidated how the smallest chiral auxiliary at the HAB periphery progressively and cooperatively boosts the overall chiroptical properties and also how subtle changes in temperature and solvent structure affect the propeller dynamics and thus the chiroptical responses. The unique features of propeller-chiral HABs further enabled us to switch on/off their circularly polarized luminescence.
@article{kosaka2018solvent, title = {Solvent and temperature effects on dynamics and chiroptical properties of propeller chirality and toroidal interaction of hexaarylbenzenes}, author = {Kosaka, Tomoyo and Iwai, Satono and Inoue, Yoshihisa and Moriuchi, Toshiyuki and Mori, Tadashi}, journal = {The Journal of Physical Chemistry A}, volume = {122}, issue = {37}, pages = {7455--7463}, year = {2018}, publisher = {ACS Publications}, doi = {10.1021/acs.jpca.8b06535}, url = {https://doi.org/10.1021/acs.jpca.8b06535}, dimensions = {true}, tab = {paper}, } -
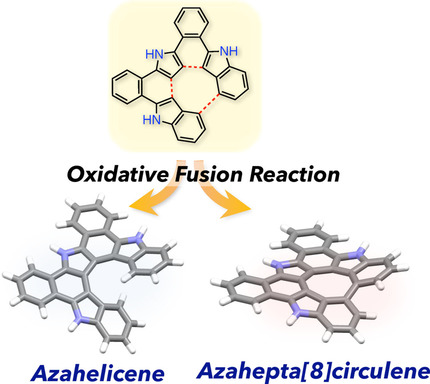 Synthesis, structures, and optical properties of azahelicene derivatives and unexpected formation of azahepta [8] circulenesFengkun Chen, Takayuki Tanaka*, Tadashi Mori, and Atsuhiro Osuka*Chem. Eur. J., 2018, 24, 7489–7497.
Synthesis, structures, and optical properties of azahelicene derivatives and unexpected formation of azahepta [8] circulenesFengkun Chen, Takayuki Tanaka*, Tadashi Mori, and Atsuhiro Osuka*Chem. Eur. J., 2018, 24, 7489–7497.Polycyclic heteroaromatic compounds including pyrrole units are promising functional scaffolds owing to their electron-rich nature, bright fluorescence, and applicability to anion recognition at the pyrrolic hydrogen atom. We report herein the effective synthesis of pseudo-aza[5]helicene and aza[7]helicene derivatives, and unexpected formation of azahepta[8]circulenes by oxidative fusion reactions. By choosing reaction conditions and peripheral substituents attached at the terminal indole moieties, we obtained aza[7]helicenes 10 a–c and azahepta[8]circulenes 11 a,c selectively in moderate to good yields. Their solid-state structures have been revealed by X-ray diffraction analysis. UV/Vis absorption, emission, and cyclic voltammetry of these compounds were studied in comparison with those of previously reported tetraaza[8]circulene (TA8C), a symmetric and planar molecule. Furthermore, the enantiomeric separation of dimethyl-substituted aza[7]helicene 10 b was achieved, and the racemization kinetics have been elucidated both theoretically and experimentally. This work illustrates a wealth of advantages of pyrrole incorporation into the polycyclic aromatic scaffolds in terms of synthetic aspects, structural variation, and optical tuning.
@article{chen2018synthesis, title = {Synthesis, structures, and optical properties of azahelicene derivatives and unexpected formation of azahepta [8] circulenes}, author = {Chen, Fengkun and Tanaka, Takayuki and Mori, Tadashi and Osuka, Atsuhiro}, journal = {Chem. Eur. J.}, volume = {24}, issue = {29}, pages = {7489--7497}, year = {2018}, publisher = {Wiley Online Library}, doi = {10.1002/chem.201800617}, url = {https://doi.org/10.1002/chem.201800617}, dimensions = {true}, tab = {paper}, } -
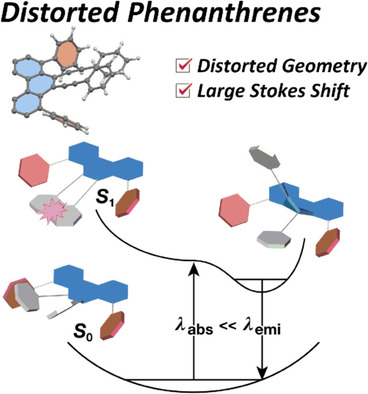 1, 8-Diphenyl-9, 10-Bis (arylethynyl) phenanthrenes: Synthesis, Distorted Structure, and Optical PropertiesAkihito Konishi*, Atsushi Morinaga, Gaku Fukuhara, Masaki Nishijima, Tadashi Mori, Toshiyuki Kida, and Makoto Yasuda*Chem. Eur. J., 2018, 24, 6625–6631.
1, 8-Diphenyl-9, 10-Bis (arylethynyl) phenanthrenes: Synthesis, Distorted Structure, and Optical PropertiesAkihito Konishi*, Atsushi Morinaga, Gaku Fukuhara, Masaki Nishijima, Tadashi Mori, Toshiyuki Kida, and Makoto Yasuda*Chem. Eur. J., 2018, 24, 6625–6631.The synthesis and optical properties of 1,8-diphenyl-9,10-bis(arylethynyl)phenanthrenes, which are distorted phenanthrenes, are reported. The presence of the two phenyl groups at the 1,8-positions of phenanthrene significantly distorts the molecular geometries, as was evidenced by X-ray crystallography. The congested substitution pattern in the K region results in a distorted aromatic framework, which leads to a redshift in the emission spectrum. These observations are in stark contrast to 9,10-bis(phenylethynyl)phenanthrene with no phenyl groups at the 1,8-positions. A large Stokes shift suggested extensive structural relaxation between the phenyl and arylethynyl units in the excited state, which was supported by theoretical calculations.
@article{konishi20181, title = {1, 8-Diphenyl-9, 10-Bis (arylethynyl) phenanthrenes: Synthesis, Distorted Structure, and Optical Properties}, author = {Konishi, Akihito and Morinaga, Atsushi and Fukuhara, Gaku and Nishijima, Masaki and Mori, Tadashi and Kida, Toshiyuki and Yasuda, Makoto}, journal = {Chem. Eur. J.}, volume = {24}, issue = {25}, pages = {6625--6631}, year = {2018}, publisher = {Wiley Online Library}, doi = {10.1002/chem.201800617}, url = {https://doi.org/10.1002/chem.201800617}, dimensions = {true}, tab = {paper}, } -
 Circularly polarized luminescence and circular dichroisms in small organic molecules: correlation between excitation and emission dissymmetry factorsHiroki Tanaka, Yoshihisa Inoue, and Tadashi Mori*ChemPhotoChem, 2018, 2, 386–402.
Circularly polarized luminescence and circular dichroisms in small organic molecules: correlation between excitation and emission dissymmetry factorsHiroki Tanaka, Yoshihisa Inoue, and Tadashi Mori*ChemPhotoChem, 2018, 2, 386–402.Prompted by the recent rapid growth of interest in circularly polarized luminescence (CPL) of organic molecules, we have collected all the reliable CPL, as well as the corresponding circular dichroism (CD), data measured in fluid solutions. To analyze the correlation between CPL and CD, we employed the absorption and luminescence dissymmetry factors (gabs and glum) of the π–π* transition reported for chiral organic molecules of various categories, including planar chiral cyclophanes and helicenes, axially chiral biaryls and spiro compounds, and point- and axially chiral BODIPY derivatives. In rigid π-systems, the absorption and fluorescence spectra are often mirror images of each other with a small Stokes shift, reflecting the minimal conformational relaxation in the emissive excited state, which should also affect the chiroptical properties in the excited state and be better sensed by CPL. However, no comprehensive efforts have hitherto been made to correlate the two relevant chiroptical properties, i.e. CPL versus CD, and also to quantitatively elucidate the effects of conformational relaxation in the excited state on the CPL behavior. The global linear regression analysis of all the reported gabs and glum values, though fairly scattered (see TOC), led us to a quantitative relationship: |glum|=0.81×|gabs| (r2=0.60), which demonstrates that the CPL dissymmetry factor is proportional to, and smaller than, the CD dissymmetry factor. A closer look revealed that the slope of the plot, or the proportional coefficient, is a critical function of the class of compounds, varying from 0.99 for cyclophanes to 0.93 for biaryls, to 0.77 for BODIPYs, and then to 0.61 for helicenes/helicenoids. The scattered glum–gabs plot and the general trend glum≤gabs appear to be inherent to the CPL of organic molecules in their isolated states, originating from the conformational flexibility, vibrational contribution, and Stokes shift that differ in each category.
@article{tanaka2018circularly, title = {Circularly polarized luminescence and circular dichroisms in small organic molecules: correlation between excitation and emission dissymmetry factors}, author = {Tanaka, Hiroki and Inoue, Yoshihisa and Mori, Tadashi}, journal = {ChemPhotoChem}, volume = {2}, issue = {5}, pages = {386--402}, year = {2018}, publisher = {Wiley Online Library}, doi = {10.1002/cptc.201800015}, url = {https://doi.org/10.1002/cptc.201800015}, dimensions = {true}, tab = {review}, } -
 Entropy-Driven Diastereoselectivity Improvement in the Paternò–Büchi Reaction of 1-Naphthyl Aryl Ethenes with a Chiral Cyanobenzoate through Remote AlkylationKeisuke Nagasaki, Yoshihisa Inoue, and Tadashi Mori*Angew. Chem. Int. Ed., 2018, 57, 4880–4885.
Entropy-Driven Diastereoselectivity Improvement in the Paternò–Büchi Reaction of 1-Naphthyl Aryl Ethenes with a Chiral Cyanobenzoate through Remote AlkylationKeisuke Nagasaki, Yoshihisa Inoue, and Tadashi Mori*Angew. Chem. Int. Ed., 2018, 57, 4880–4885.The precise stereocontrol of photocycloaddition reactions is still a significant challenge owing to their mechanistic complexity and the involvement of highly reactive and short-lived intermediates. Attempts have hitherto been made through structural modifications, mostly by introducing steric conflicts, to increase the difference between the enthalpic barriers. Herein, we show that entropy plays a crucial role in influencing the diastereoselectivity of a Paternò–Büchi reaction. Remote meta alkylation of the donor caused nominal changes in its photophysical properties as well as those of the exciplexes derived thereof. Nevertheless, the diastereomeric excess of the oxetane product was greatly improved by about 40%. This enhancement, which is not accompanied by any significant changes in the photophysical properties, is difficult to rationalize by conventional enthalpic control concepts based on repulsive steric and/or attractive intermolecular interactions as well as electronic perturbations. Differential activation parameters and compensatory enthalpy–entropy relationships revealed that the diastereoselectivity enhancement is not simply enthalpic but also entropic in origin.
@article{nagasaki2018entropy, title = {Entropy-Driven Diastereoselectivity Improvement in the Paternò--Büchi Reaction of 1-Naphthyl Aryl Ethenes with a Chiral Cyanobenzoate through Remote Alkylation}, author = {Nagasaki, Keisuke and Inoue, Yoshihisa and Mori, Tadashi}, journal = {Angew. Chem. Int. Ed.}, volume = {57}, issue = {18}, pages = {4880--4885}, year = {2018}, publisher = {Wiley Online Library}, doi = {10.1002/anie.201801330}, url = {https://doi.org/10.1002/anie.201801330}, dimensions = {true}, tab = {paper}, } -
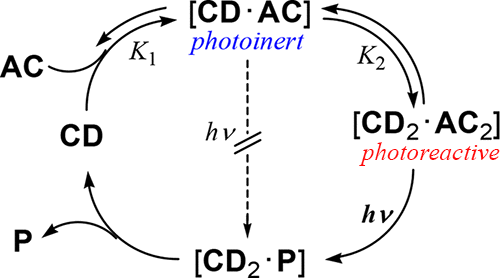 Supramolecular photochirogenesis driven by higher-order complexation: enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate to slipped cyclodimers via a 2: 2 complex with β-cyclodextrinXueqin Wei, Wanhua Wu, Ryohei Matsushita, Zhiqiang Yan, Dayang Zhou, Jason J Chruma, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cheng Yang*J. Am. Chem. Soc., 2018, 140, 3959–3974.
Supramolecular photochirogenesis driven by higher-order complexation: enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate to slipped cyclodimers via a 2: 2 complex with β-cyclodextrinXueqin Wei, Wanhua Wu, Ryohei Matsushita, Zhiqiang Yan, Dayang Zhou, Jason J Chruma, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cheng Yang*J. Am. Chem. Soc., 2018, 140, 3959–3974.Chiral slipped 5,8:9′,10′-cyclodimers were preferentially produced over classical 9,10:9′,10′-cyclodimers upon supramolecular photocyclodimerization of 2-anthracenecarboxylate (AC) mediated by β-cyclodextrin (β-CD). This photochirogenic route to the slipped cyclodimers, exclusively head-to-tail (HT) and highly enantioselective, has long been overlooked in foregoing studies but is dominant in reality and is absolutely supramolecularly activated by 2:2 complexation of AC with β-CD. The intricate structural and photophysical aspects of this higher-order complexation-triggered process have been comprehensively elucidated, while the absolute configurations of the slipped cyclodimers have been unambiguously assigned by comparing the experimental and theoretical circular dichroism spectra. In the 2:2 complex, two ACs packed in a dual β-CD capsule are not fully overlapped with each other but are only partially stacked in a slipped anti- or syn-HT manner. Hence, they do not spontaneously cyclodimerize upon photoexcitation but instead emit long-lived excimer fluorescence at wavelengths slightly longer than the monomer fluorescence, indicating that the slipped excimer is neither extremely reactive nor completely relaxed in conformation and energy. Because of the slipped conformation of the AC pair in the soft capsule, the subsequent photocyclodimerization becomes manipulable by various internal or external factors, such as temperature, pressure, added salt, and host modification, enabling us to exclusively obtain the slipped cyclodimers with high regio- and enantioselectivities. In this supramolecularly driven photochirogenesis, the dual β-CD capsule functions as a chiral organophotocatalyst to trigger and accelerate the nonclassical photochirogenic route to slipped cyclodimers by preorganizing the conformation of the encapsulated AC pair, formally mimicking a catalytic antibody.
@article{wei2018supramolecular, title = {Supramolecular photochirogenesis driven by higher-order complexation: enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate to slipped cyclodimers via a 2: 2 complex with β-cyclodextrin}, author = {Wei, Xueqin and Wu, Wanhua and Matsushita, Ryohei and Yan, Zhiqiang and Zhou, Dayang and Chruma, Jason J and Nishijima, Masaki and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa and Yang, Cheng}, journal = {J. Am. Chem. Soc.}, volume = {140}, issue = {11}, pages = {3959--3974}, year = {2018}, publisher = {ACS Publications}, doi = {10.1021/jacs.7b12085}, url = {https://doi.org/10.1021/jacs.7b12085}, dimensions = {true}, tab = {paper}, } -
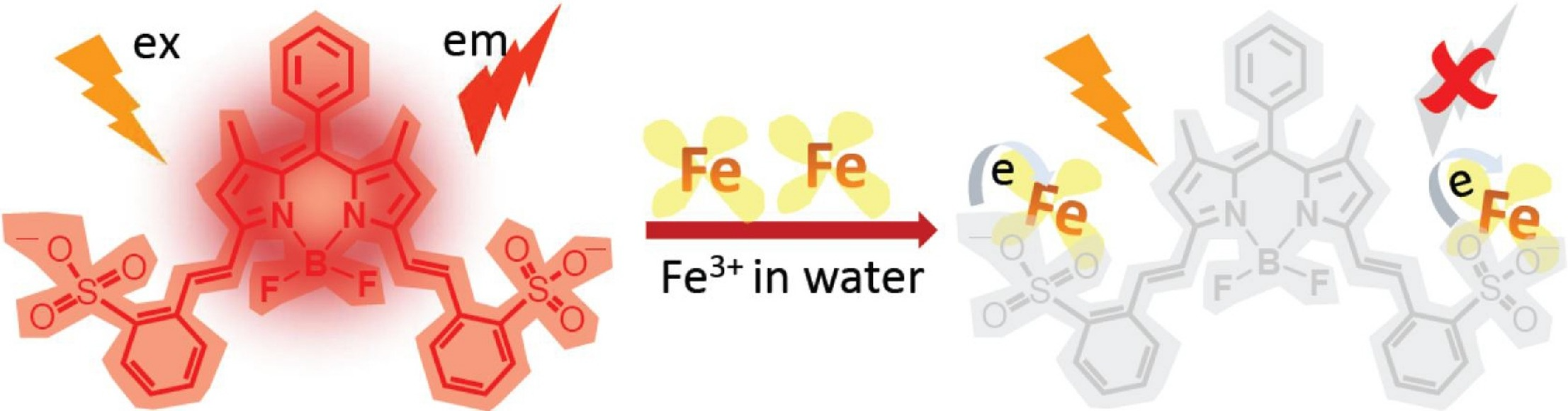 A BODIPY-based near infrared fluorescent probe for Fe3+ in waterJiecheng Ji, Syama Sundar Chereddy, Yi Ren, Xingming Chen, Dan Su, Zhihui Zhong, Tadashi Mori, Yoshihisa Inoue*, Wanhua Wu, and Cheng Yang*J. Photochem. Photobiol. A: Chem., 2018, 355, 78–83.
A BODIPY-based near infrared fluorescent probe for Fe3+ in waterJiecheng Ji, Syama Sundar Chereddy, Yi Ren, Xingming Chen, Dan Su, Zhihui Zhong, Tadashi Mori, Yoshihisa Inoue*, Wanhua Wu, and Cheng Yang*J. Photochem. Photobiol. A: Chem., 2018, 355, 78–83.For highly sensitive and selective detection of biologically important Fe3+ in aqueous media, a novel near-infrared (NIR) fluorescent probe (λem = 627 nm; ΦF = 0.47) based on boron-dipyrromethene (BODIPY) was developed. Employing a pair of 2-sulfonatostyryl sidearms attached to the BODIPY core as a reversible chelator specific to Fe3+, the sensor achieved a high selectivity toward Fe3+ over a wide range of metal ions, including Li+, Na+, K+, Mg2+, Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Al3+, and Cr3+. Furthermore, the quenched NIR fluorescence was immediately recovered upon addition of sodium ascorbate as a reductant or EDTA as a stronger chelator.
@article{ji2018bodipy, title = {A BODIPY-based near infrared fluorescent probe for Fe3+ in water}, author = {Ji, Jiecheng and Chereddy, Syama Sundar and Ren, Yi and Chen, Xingming and Su, Dan and Zhong, Zhihui and Mori, Tadashi and Inoue, Yoshihisa and Wu, Wanhua and Yang, Cheng}, journal = {J. Photochem. Photobiol. A: Chem.}, volume = {355}, pages = {78--83}, year = {2018}, publisher = {Elsevier}, doi = {10.1016/j.jphotochem.2017.10.043}, url = {https://doi.org/10.1016/j.jphotochem.2017.10.043}, dimensions = {true}, tab = {paper}, } -
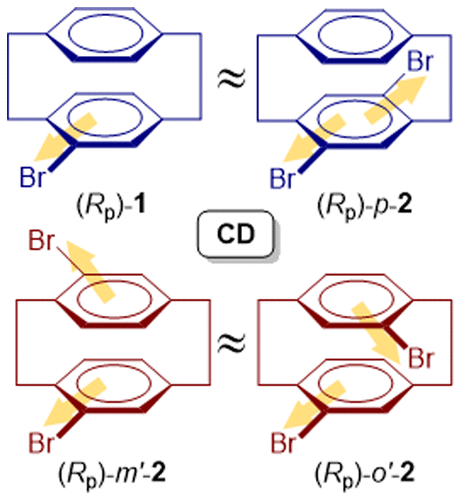 Circular Dichroisms of Mono-and Dibromo [2.2] paracyclophanes: A Combined Experimental and Theoretical StudyMitsunobu Toda, Yoshihisa Inoue, and Tadashi Mori*ACS Omega, 2018, 3, 22–29.
Circular Dichroisms of Mono-and Dibromo [2.2] paracyclophanes: A Combined Experimental and Theoretical StudyMitsunobu Toda, Yoshihisa Inoue, and Tadashi Mori*ACS Omega, 2018, 3, 22–29.Circular dichroisms (CDs) of planar chiral 4-bromo[2.2]paracyclophane (1) and three isomeric dibromo[2.2]paracyclophanes (p-2, m′-2, and o′-2) were investigated experimentally and theoretically. They all exhibited strong multisignate Cotton effects (CEs) at the 1Lb, 1La, and 1B transitions of the component (bromo)benzene chromophore and were comparable to each other. For all of the cyclophanes examined, the enantiomer that eluted earlier from a chiral high-performance liquid chromatography column (Chiralcel IA or IB) exhibited negative and positive CEs at the 1Lb and 1La bands, respectively, which were followed by a more complicated pattern of CDs at the higher-energy bands. These CD features were well reproduced by quantum chemical calculations, allowing us to unambiguously assign the absolute configurations of the first-eluted enantiomers as Rp in all of the cases examined. Interestingly, the CDs of 1 and 2, although largely comparable in shape, were still sensitive to the number and pattern of bromine substitution, showing closer resemblance between m-2 and o-2 and between p-2 and 1. The theoretical calculations also reproduced successfully these spectral resemblance between them. The anisotropy (g) factors for the 1Lb bands of these cyclophanes were considerably large (∼10–2), whereas those for the 1La band were conventional in the order of 10–3. In addition, a weak CE was observed in the low-energy region at around 320 nm, which turned out to originate from the interplanar interaction and is hence assigned to the “cyclophane band”. The experimental g factors of this band were fairly large in the order of 10–2, but the computation turned out to be quite challenging and were less well reproduced theoretically, ascribable to the forbidden nature of the transition.
@article{toda2018circular, title = {Circular Dichroisms of Mono-and Dibromo [2.2] paracyclophanes: A Combined Experimental and Theoretical Study}, author = {Toda, Mitsunobu and Inoue, Yoshihisa and Mori, Tadashi}, journal = {ACS Omega}, volume = {3}, issue = {1}, pages = {22--29}, year = {2018}, publisher = {ACS Publications}, doi = {10.1021/acsomega.7b01642}, url = {https://doi.org/10.1021/acsomega.7b01642}, dimensions = {true}, tab = {paper}, } -
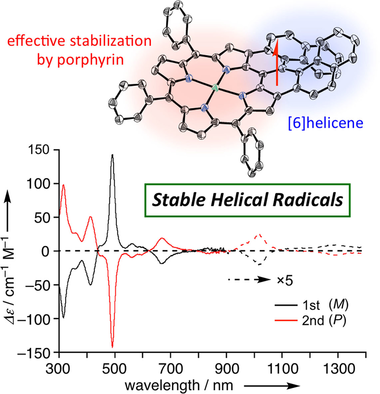 Porphyrin-Based Air-Stable Helical RadicalsKenichi Kato, Ko Furukawa*, Tadashi Mori, and Atsuhiro Osuka*Chem. Eur. J., 2018, 24, 572–575.
Porphyrin-Based Air-Stable Helical RadicalsKenichi Kato, Ko Furukawa*, Tadashi Mori, and Atsuhiro Osuka*Chem. Eur. J., 2018, 24, 572–575.Stable helical radicals are promising multi-functional molecules in light of intriguing magnetic and chiroptical properties. Attempts were made to extend diphenylmethyl-fused NiII porphyrin radical to helical system as the first air-stable organic neutral helical radicals. Intramolecular Pd-catalyzed twofold C−H arylation of methyl- or methoxy-introduced meso-diphenylmethyl NiII porphyrins gave a mixture of the target and rearranged radicals. Oxidative fusion reaction of meso-(bis(1-naphthyl)methyl) NiII porphyrins provided doubly fused NiII porphyrin radicals. One of the helical radicals was separated into enantiomers that showed mirror-image circular dichroism (CD) spectra up to 1300 nm. The helical dinaphthylmethyl-fused NiII porphyrin radical displayed solid-state magnetic property mostly arising from monomeric radicals, different from the parent diphenylmethyl-fused NiII porphyrin radical that showed antiferromagnetic coupling due to π-stacked pairing.
@article{kato2018porphyrin, title = {Porphyrin-Based Air-Stable Helical Radicals}, author = {Kato, Kenichi and Furukawa, Ko and Mori, Tadashi and Osuka, Atsuhiro}, journal = {Chem. Eur. J.}, volume = {24}, number = {3}, pages = {572--575}, year = {2018}, publisher = {Wiley Online Library}, doi = {10.1002/chem.201705291}, url = {https://doi.org/10.1002/chem.201705291}, dimensions = {true}, tab = {paper}, } -
 Preface for special issue on photosynergeticsVasudevanpillai Biju*, Tadashi Mori*, Tsuyoshi Asahi*, and Hiroshi Miyasaka*J. Photochem. Photobiol. C: Photochem. Rev., 2018, 34, 1.
Preface for special issue on photosynergeticsVasudevanpillai Biju*, Tadashi Mori*, Tsuyoshi Asahi*, and Hiroshi Miyasaka*J. Photochem. Photobiol. C: Photochem. Rev., 2018, 34, 1.Molecules in the electronic excited state photo-energy conversion natural artificial photosynthesis so forth. For molecules in condensed phase however three general restrictions limit the efficient utilization of light energy. First molecules in higher electronically-excited states rapidly relax to the lowest excited electronic state (Kasha’s rule) extinguishing some portion of the absorbed photon energy. Second a large number of molecules excited in assemblies undergo fast annihilation leaving only a small fraction in the excited state leading to the loss of a major fraction of the number of photons absorbed by the assemblies. Third the electronic state accessible through one-photon absorption is limited by the optical selection rule denying access to various dark electronic excited states of molecules.
@article{biju2018preface, title = {Preface for special issue on photosynergetics}, author = {Biju, Vasudevanpillai and Mori, Tadashi and Asahi, Tsuyoshi and Miyasaka, Hiroshi}, journal = {J. Photochem. Photobiol. C: Photochem. Rev.}, volume = {34}, pages = {1}, year = {2018}, publisher = {Elsevier BV}, doi = {10.1016/j.jphotochemrev.2018.02.003.}, url = {https://doi.org/10.1016/j.jphotochemrev.2018.02.003.}, dimensions = {true}, tab = {paper}, } - Propeller ChiralityTadashi Mori*Kokagaku, 2018, 49, 163–166.
@article{mori2018propeller, title = {Propeller Chirality}, author = {Mori, Tadashi}, journal = {Kokagaku}, volume = {49}, issue = {3}, pages = {163--166}, year = {2018} }