articles in 2017
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
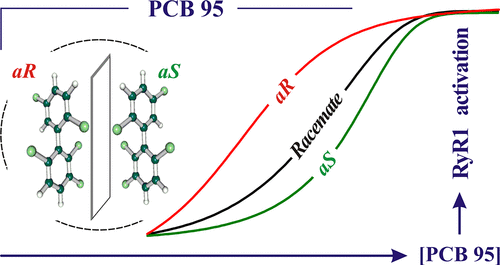 Enantioselectivity of 2, 2′, 3, 5′, 6-pentachlorobiphenyl (PCB 95) atropisomers toward ryanodine receptors (RyRs) and their influences on hippocampal neuronal networksWei Feng, Jing Zheng, Gaëlle Robin, Yao Dong, Makoto Ichikawa, Yoshihisa Inoue, Tadashi Mori, Takeshi Nakano, and Isaac N Pessah*Environ. Sci. Technol., 2017, 51, 14406–14416.
Enantioselectivity of 2, 2′, 3, 5′, 6-pentachlorobiphenyl (PCB 95) atropisomers toward ryanodine receptors (RyRs) and their influences on hippocampal neuronal networksWei Feng, Jing Zheng, Gaëlle Robin, Yao Dong, Makoto Ichikawa, Yoshihisa Inoue, Tadashi Mori, Takeshi Nakano, and Isaac N Pessah*Environ. Sci. Technol., 2017, 51, 14406–14416.Nineteen ortho-substituted PCBs are chiral and found enantioselectively enriched in ecosystems. Their differential actions on biological targets are not understood. PCB 95 (2,2′,3,5′,6-pentachlorobiphenyl), a chiral PCB of current environmental relevance, is among the most potent toward modifying ryanodine receptors (RyR) function and Ca2+ signaling. PCB 95 enantiomers are separated and assigned aR- and aS-PCB 95 using three chiral-column HPLC and circular dichroism spectroscopy. Studies of RyR1-enriched microsomes show aR-PCB 95 with >4× greater potency (EC50 = 0.20 ± 0.05 μM), ∼ 1.3× higher efficacy (Bmax = 3.74 ± 0.07 μM) in [3H]Ryanodine-binding and >3× greater rates (R = 7.72 ± 0.31 nmol/sec/mg) of Ca2+ efflux compared with aS-PCB 95, whereas racemate has intermediate activity. aR-PCB 95 has modest selectivity for RyR2, and lower potency than racemate toward the RyR isoform mixture in brain membranes. Chronic exposure of hippocampal neuronal networks to nanomolar PCB 95 during a critical developmental period shows divergent influences on synchronous Ca2+ oscillation (SCO): rac-PCB 95 increasing and aR-PCB 95 decreasing SCO frequency at 50 nM, although the latter’s effects are nonmonotonic at higher concentration. aS-PCB95 shows the greatest influence on inhibiting responses to 20 Hz electrical pulse trains. Considering persistence of PCB 95 in the environment, stereoselectivity toward RyRs and developing neuronal networks may clarify health risks associated with enantioisomeric enrichment of PCBs.
@article{feng2017enantioselectivity, title = {Enantioselectivity of 2, 2′, 3, 5′, 6-pentachlorobiphenyl (PCB 95) atropisomers toward ryanodine receptors (RyRs) and their influences on hippocampal neuronal networks}, author = {Feng, Wei and Zheng, Jing and Robin, Gaëlle and Dong, Yao and Ichikawa, Makoto and Inoue, Yoshihisa and Mori, Tadashi and Nakano, Takeshi and Pessah, Isaac N}, journal = {Environ. Sci. Technol.}, volume = {51}, issue = {24}, pages = {14406--14416}, year = {2017}, publisher = {ACS Publications}, doi = {10.1021/acs.est.7b04446}, url = {https://doi.org/10.1021/acs.est.7b04446}, dimensions = {true}, tab = {paper}, } -
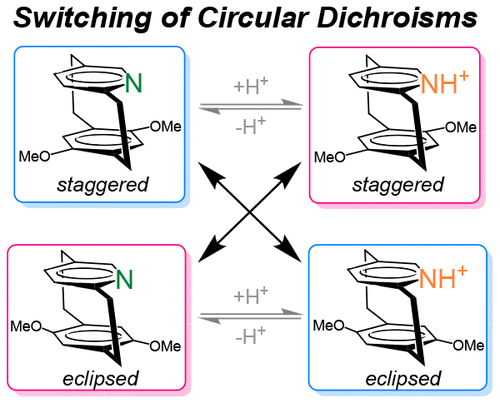 A combined experimental and theoretical study on the circular dichroism of staggered and eclipsed forms of dimethoxy [2.2]-,[3.2]-, and [3.3] pyridinophanes and their protonated formsAkinori Shimizu, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. A, 2017, 121, 8389–8398.
A combined experimental and theoretical study on the circular dichroism of staggered and eclipsed forms of dimethoxy [2.2]-,[3.2]-, and [3.3] pyridinophanes and their protonated formsAkinori Shimizu, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. A, 2017, 121, 8389–8398.The circular dichroisms (CDs) of dimethoxy[2.2]-, [3.2]-, and [3.3]pyridinophanes and their protonated forms were investigated experimentally and theoretically. Characteristic multisignate Cotton effects (CEs), typical for planar chiral cyclophane derivatives, were observed. The CD spectral pattern was quite comparable for the staggered forms of [2.2]-, [3.2]-, and [3.3]cyclophanes, but significantly differed for the eclipsed forms. More interestingly, the patterns resembled, but the CE signs were practically opposite between staggered and eclipsed [2.2]pyridinophanes. Upon protonation, the signs of most CEs were inverted in both forms of cyclophanes, due to the reversal of dipole moment in the pyridine against the pyridinium moiety. Such a change in CD spectrum upon protonation was not apparent in [3.2]pyridinophane, and the CD spectral behavior was more complex in [3.3]pyridinophanes. The variation of CD caused by the protonation/deprotonation process was temperature-dependent and hence utilized as a thermal sensor. The protonated forms of the homologous pyridinophanes with different tether lengths in staggered and eclipsed forms served as a model system for systematically studying the cation−π interaction and its effects on chiroptical properties. A steady increase of electronic interaction became apparent for the smaller-sized cyclophanes from the increased excitation energy and electronic coupling element of the charge-transfer (CT) band, while the observed CE at the CT band was a more complex function of the original transition dipole of donor/acceptor pair and linker atoms, as well as the strength of the electronic interaction.
@article{shimizu2017combined, title = {A combined experimental and theoretical study on the circular dichroism of staggered and eclipsed forms of dimethoxy [2.2]-,[3.2]-, and [3.3] pyridinophanes and their protonated forms}, author = {Shimizu, Akinori and Inoue, Yoshihisa and Mori, Tadashi}, journal = {J. Phys. Chem. A}, volume = {121}, issue = {44}, pages = {8389--8398}, year = {2017}, publisher = {ACS Publications}, doi = {10.1021/acs.jpca.7b08623}, url = {https://doi.org/10.1021/acs.jpca.7b08623}, dimensions = {true}, tab = {paper}, } -
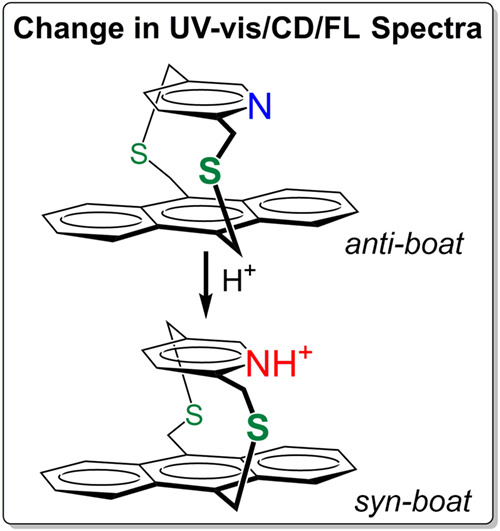 Chiroptical properties of dithia [3.3] cyclophanes composed of anthracene and pyridine/pyridinium moieties: A combined experimental and theoretical studyAkinori Shimizu, Keisuke Nagasaki, Yoshihisa Inoue, and Tadashi Mori*Chirality, 2017, 121, 8389–8398.
Chiroptical properties of dithia [3.3] cyclophanes composed of anthracene and pyridine/pyridinium moieties: A combined experimental and theoretical studyAkinori Shimizu, Keisuke Nagasaki, Yoshihisa Inoue, and Tadashi Mori*Chirality, 2017, 121, 8389–8398.Circular dichroisms (CDs) of neutral and protonated [3.3]anthracenopyridinophane (1 and 1-H+) were investigated experimentally and theoretically. Introducing an anthracene moiety with extended conjugation affected the cyclophane structure with the bent angles being appreciably reduced from those of parent [3.3]pyridinophane. The Cotton effects (CEs) observed at the 1Bb band for both 1 and 1-H+ were fairly strong and apparently bisignate, which, however, turned out not to be a simple exciton couplet but to be composed of multiple transitions. In contrast, the CEs were much weaker in the 1La band region. The spectral changes upon protonation were less significant compared with the parent pyridinophane, being dominated by the local transitions of anthracene. Nevertheless, the CD spectra of 1 and 1-H+ were well reproduced by theoretical calculations to allow us an unambiguous absolute configuration determination of the first high-performance liquid chromatography (HPLC) elute (from Chiralcel IB column) as Sp. The transannular interactions between the anthracene and pyridine/pyridinium units were examined by UV-vis and fluorescence spectroscopy to reveal a charge-transfer (CT) band in the low-energy region, particularly for 1-H+. Despite the comparable CT interactions, the CE at the CT band was much stronger for the anthracenopyridinophane than for the parent pyridinophane, affording an anisotropy (g) factor as large as 4 × 10-3.
@article{shimizu2017chiroptical, title = {Chiroptical properties of dithia [3.3] cyclophanes composed of anthracene and pyridine/pyridinium moieties: A combined experimental and theoretical study}, author = {Shimizu, Akinori and Nagasaki, Keisuke and Inoue, Yoshihisa and Mori, Tadashi}, journal = {Chirality}, volume = {121}, issue = {44}, pages = {8389--8398}, year = {2017}, publisher = {ACS Publications}, doi = {10.1002/chir.22740}, url = {https://doi.org/10.1002/chir.22740}, dimensions = {true}, tab = {paper}, } -
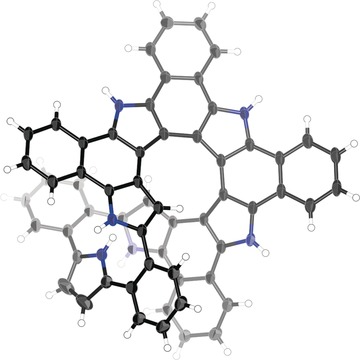 Closed Pentaaza [9] helicene and Hexathia [9]/[5] helicene: Oxidative Fusion Reactions of ortho-Phenylene-Bridged Cyclic Hexapyrroles and HexathiophenesFengkun Chen, Takayuki Tanaka*, Yong Seok Hong, Tadashi Mori, Dongho Kim*, and Atsuhiro Osuka*Angew. Chem. Int. Ed., 2017, 129, 14880–14885.
Closed Pentaaza [9] helicene and Hexathia [9]/[5] helicene: Oxidative Fusion Reactions of ortho-Phenylene-Bridged Cyclic Hexapyrroles and HexathiophenesFengkun Chen, Takayuki Tanaka*, Yong Seok Hong, Tadashi Mori, Dongho Kim*, and Atsuhiro Osuka*Angew. Chem. Int. Ed., 2017, 129, 14880–14885.Oxidative fusion reactions of ortho-phenylene-bridged cyclic hexapyrroles and hexathiophenes furnished novel closed helicenes in a selective manner. X-Ray diffraction analysis unambiguously revealed the structures to be a closed pentaaza[9]helicene, the longest azahelicene reported so far, and an unexpected double-helical structure of hexathia[9]/[5]helicene, whose formation was assumed to result from multiple oxidative fusion along with a 1,2-aryl shift. The pentaaza[9]helicene exhibited well-defined emission with high fluorescence quantum yield (ΦF=0.31) among the known [9]helicenes. Chiral resolution of the racemic pentaaza[9]helicene and hexathia[9]/[5]helicene were achieved by chiral-phase HPLC and the enantiomers were characterized by circular dichroism spectra and DFT calculations.
@article{chen2017closed, title = {Closed Pentaaza [9] helicene and Hexathia [9]/[5] helicene: Oxidative Fusion Reactions of ortho-Phenylene-Bridged Cyclic Hexapyrroles and Hexathiophenes}, author = {Chen, Fengkun and Tanaka, Takayuki and Hong, Yong Seok and Mori, Tadashi and Kim, Dongho and Osuka, Atsuhiro}, journal = {Angew. Chem. Int. Ed.}, volume = {129}, issue = {46}, pages = {14880--14885}, year = {2017}, publisher = {Wiley Online Library}, doi = {10.1002/anie.201708429}, url = {https://doi.org/10.1002/anie.201708429}, dimensions = {true}, tab = {paper}, } -
 Intense redox-driven chiroptical switching with a 580 mV hysteresis actuated through reversible dimerization of an azoniaheliceneJochen R Brandt, Lubomír Pospíšil, Lucie Bednárová, Rosenildo Correa Costa, Andrew JP White, Tadashi Mori, Filip Teplý, and Matthew J Fuchter*Chem. Commun., 2017, 53, 9059–9062.
Intense redox-driven chiroptical switching with a 580 mV hysteresis actuated through reversible dimerization of an azoniaheliceneJochen R Brandt, Lubomír Pospíšil, Lucie Bednárová, Rosenildo Correa Costa, Andrew JP White, Tadashi Mori, Filip Teplý, and Matthew J Fuchter*Chem. Commun., 2017, 53, 9059–9062.Electrochemical reduction of an azoniahelicene affords a dimer, accompanied by a strong change in the electronic circular dichroism. The fast dimerisation event leads to a >500 mV shift of the oxidation potential, affording a large area of bistability, where the chiroptical signal only depends on the redox history.
@article{brandt2017intense, title = {Intense redox-driven chiroptical switching with a 580 mV hysteresis actuated through reversible dimerization of an azoniahelicene}, author = {Brandt, Jochen R and Pospíšil, Lubomír and Bednárová, Lucie and da Costa, Rosenildo Correa and White, Andrew JP and Mori, Tadashi and Teplý, Filip and Fuchter, Matthew J}, journal = {Chem. Commun.}, volume = {53}, issue = {65}, pages = {9059--9062}, year = {2017}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c7cc04903j}, url = {https://doi.org/10.1039/c7cc04903j}, dimensions = {true}, tab = {paper}, } -
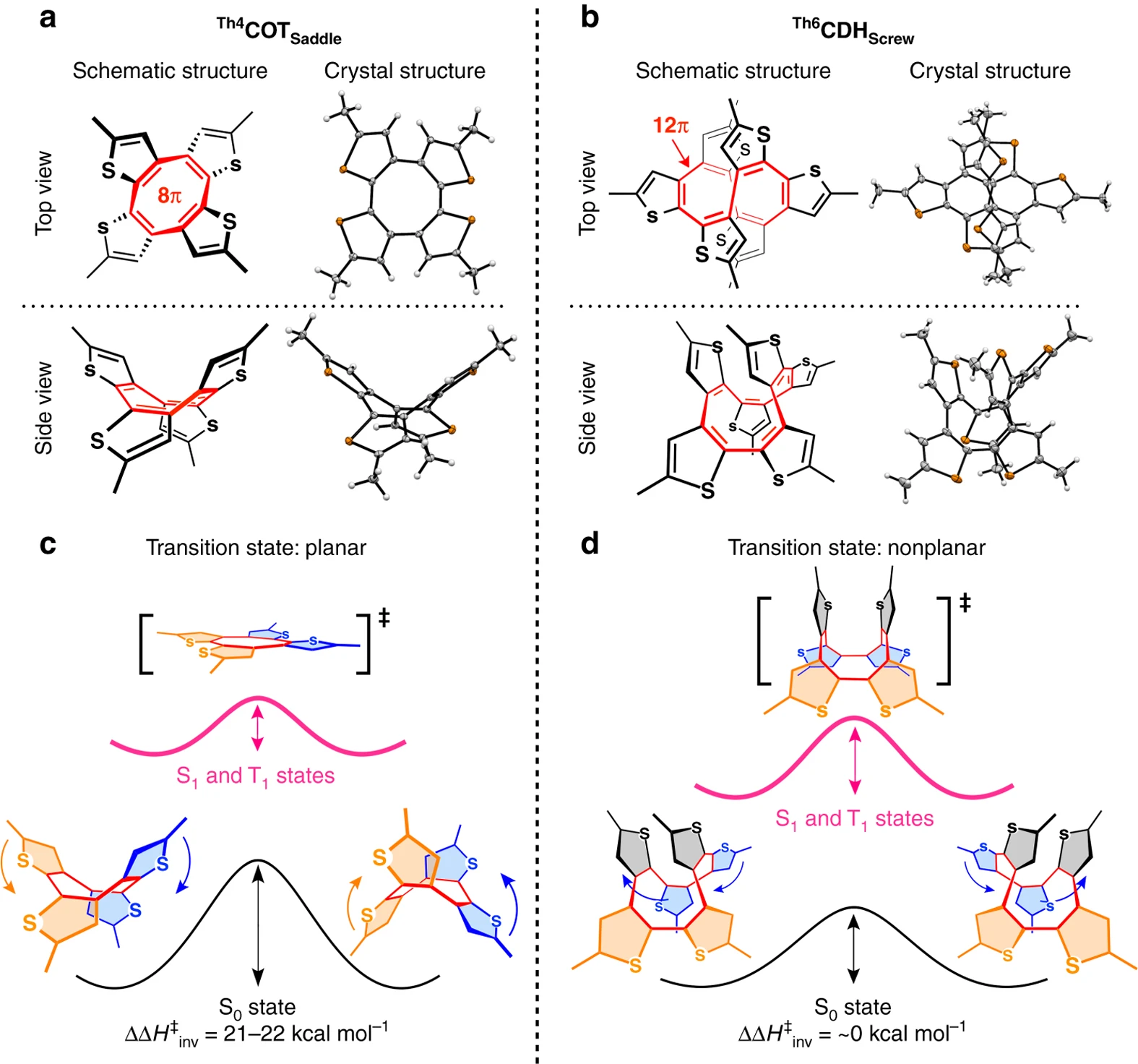 Energetics of Baird aromaticity supported by inversion of photoexcited chiral [4n] annulene derivativesMichihisa Ueda, Kjell Jorner, Young Mo Sung, Tadashi Mori, Qi Xiao, Dongho Kim, Henrik Ottosson, Takuzo Aida, and Yoshimitsu Itoh*Nat. Commun., 2017, 8, 346.
Energetics of Baird aromaticity supported by inversion of photoexcited chiral [4n] annulene derivativesMichihisa Ueda, Kjell Jorner, Young Mo Sung, Tadashi Mori, Qi Xiao, Dongho Kim, Henrik Ottosson, Takuzo Aida, and Yoshimitsu Itoh*Nat. Commun., 2017, 8, 346.For the concept of aromaticity, energetic quantification is crucial. However, this has been elusive for excited-state (Baird) aromaticity. Here we report our serendipitous discovery of two nonplanar thiophene-fused chiral [4n]annulenes Th4 COT Saddle and Th6 CDH Screw , which by computational analysis turned out to be a pair of molecules suitable for energetic quantification of Baird aromaticity. Their enantiomers were separable chromatographically but racemized thermally, enabling investigation of the ring inversion kinetics. In contrast to Th6 CDH Screw , which inverts through a nonplanar transition state, the inversion of Th4 COT Saddle , progressing through a planar transition state, was remarkably accelerated upon photoexcitation. As predicted by Baird’s theory, the planar conformation of Th4 COT Saddle is stabilized in the photoexcited state, thereby enabling lower activation enthalpy than that in the ground state. The lowering of the activation enthalpy, i.e., the energetic impact of excited-state aromaticity, was quantified experimentally to be as high as 21–22 kcal mol–1.
@article{ueda2017energetics, title = {Energetics of Baird aromaticity supported by inversion of photoexcited chiral [4n] annulene derivatives}, author = {Ueda, Michihisa and Jorner, Kjell and Sung, Young Mo and Mori, Tadashi and Xiao, Qi and Kim, Dongho and Ottosson, Henrik and Aida, Takuzo and Itoh, Yoshimitsu}, journal = {Nat. Commun.}, volume = {8}, issue = {1}, pages = {346}, year = {2017}, publisher = {Nature Publishing Group UK London}, doi = {10.1038/s41467-017-00382-1}, url = {https://doi.org/10.1038/s41467-017-00382-1}, dimensions = {true}, tab = {paper}, } -
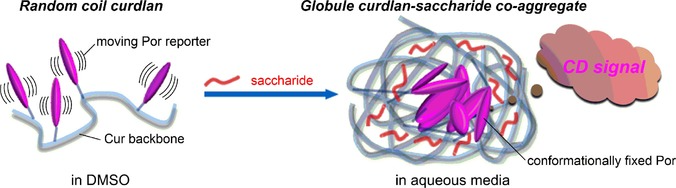 Oligosaccharide Sensing in Aqueous Media by Porphyrin–Curdlan Conjugates: A Prêt-á-Porter Rather Than Haute-Couture ApproachGaku Fukuhara*, Mayuko Sasaki, Munenori Numata, Tadashi Mori, and Yoshihisa Inoue*Chem. Eur. J., 2017, 23, 11272–11278.
Oligosaccharide Sensing in Aqueous Media by Porphyrin–Curdlan Conjugates: A Prêt-á-Porter Rather Than Haute-Couture ApproachGaku Fukuhara*, Mayuko Sasaki, Munenori Numata, Tadashi Mori, and Yoshihisa Inoue*Chem. Eur. J., 2017, 23, 11272–11278.Saccharide sensing in aqueous media is an intriguing but challenging goal in current chemistry. Herein we report the oligosaccharide-sensing behavior of newly synthesized porphyrin–curdlan conjugates, which are random coils in DMSO but become globules in aqueous solutions to induce circular dichroism (ICD) in the biologically accessible spectral region due to the conformational fixation of porphyrin reporters. The magnitude of ICD was significantly varied specifically in the presence of acarbose, a drug for type-2 diabetes, enabling us to detect the aminosaccharide at concentrations down to 200 μm. This result demonstrates that the prêt-á-porter approach, using less-defined reporter-curdlan conjugates, is more advantageous than the traditional haute-couture approach with highly sophisticated hosts in particular in oligosaccharide sensing.
@article{fukuhara2017oligosaccharide, title = {Oligosaccharide Sensing in Aqueous Media by Porphyrin--Curdlan Conjugates: A Prêt-á-Porter Rather Than Haute-Couture Approach}, author = {Fukuhara, Gaku and Sasaki, Mayuko and Numata, Munenori and Mori, Tadashi and Inoue, Yoshihisa}, journal = {Chem. Eur. J.}, volume = {23}, issue = {47}, pages = {11272--11278}, year = {2017}, publisher = {Wiley Online Library}, doi = {10.1002/chem.201701360}, url = {https://doi.org/10.1002/chem.201701360}, dimensions = {true}, tab = {paper}, } -
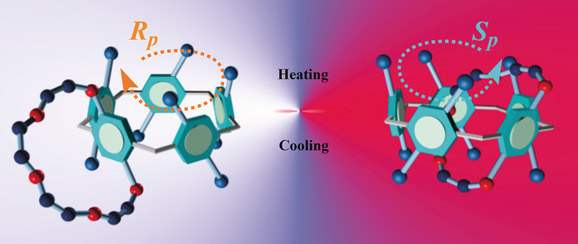 Temperature-driven planar chirality switching of a pillar [5] arene-based molecular universal jointJiabin Yao, Wanhua Wu, Wenting Liang, Yujun Feng, Dayang Zhou, Jason J Chruma, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue, and Cheng Yang*Angew. Chem. Int. Ed., 2017, 129, 6973–6977.
Temperature-driven planar chirality switching of a pillar [5] arene-based molecular universal jointJiabin Yao, Wanhua Wu, Wenting Liang, Yujun Feng, Dayang Zhou, Jason J Chruma, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue, and Cheng Yang*Angew. Chem. Int. Ed., 2017, 129, 6973–6977.The study of an enantiopure bicyclic pillar[5]arene-based molecular universal joint (MUJ) by single-crystal X-ray diffraction allowed for the first time the unequivocal assignment of the absolute configuration of a planar chiral pillar[5]arene by circular dichroism spectroscopy. Crucially, the absolute configuration of the MUJ was switched reversibly by temperature, with an accompanying sign inversion of the anisotropy factor that varied by as much as 0.03, which is the largest value ever reported. Mechanistically, the reversible chirality switching of the MUJ is driven by the threading/dethreading motion of the fused ring and hence is dependent on both the size and nature of the ring and the solvent employed, reflecting the critical balance between the self-complexation of the ring by pillar[5]arene, the solvation to the excluded ring, and the inclusion of solvent molecules in the cavity.
@article{yao2017temperature, title = {Temperature-driven planar chirality switching of a pillar [5] arene-based molecular universal joint}, author = {Yao, Jiabin and Wu, Wanhua and Liang, Wenting and Feng, Yujun and Zhou, Dayang and Chruma, Jason J and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa and Yang, Cheng}, journal = {Angew. Chem. Int. Ed.}, volume = {129}, issue = {24}, pages = {6973--6977}, year = {2017}, publisher = {Wiley Online Library}, doi = {10.1002/anie.201702542}, url = {https://doi.org/10.1002/anie.201702542}, dimensions = {true}, tab = {paper}, } -
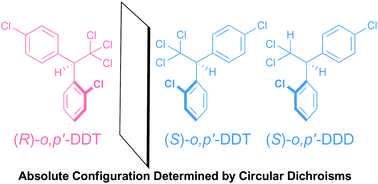 Absolute configuration determination through the unique intramolecular excitonic coupling in the circular dichroisms of o, p′-DDT and o, p′-DDD. A combined experimental and theoretical studyHiroki Tanaka, Yoshihisa Inoue, Takeshi Nakano, and Tadashi Mori*Photochem. Photobiol. Sci., 2017, 16, 606–610.
Absolute configuration determination through the unique intramolecular excitonic coupling in the circular dichroisms of o, p′-DDT and o, p′-DDD. A combined experimental and theoretical studyHiroki Tanaka, Yoshihisa Inoue, Takeshi Nakano, and Tadashi Mori*Photochem. Photobiol. Sci., 2017, 16, 606–610.Circular dichroisms (CDs) of the o,p′-isomers of 1,1,1-trichloro- and 1,1-dichloro-2,2-bis(chlorophenyl)ethanes (DDT and DDD) were investigated experimentally and theoretically. A series of strong Cotton effect peaks in a characteristic negative–negative–positive–negative, or its mirror-imaged, pattern were observed in the CD spectra of these persistent organic pollutants. The theoretical CD spectra at the SAC-CI/B95(d) and RI-CC2/def2-TZVPP levels well reproduced the experimental ones, enabling us to unambiguously assign the absolute configuration of (+)-DDT and (−)-DDD as S.
@article{tanaka2017absolute, title = {Absolute configuration determination through the unique intramolecular excitonic coupling in the circular dichroisms of o, p′-DDT and o, p′-DDD. A combined experimental and theoretical study}, author = {Tanaka, Hiroki and Inoue, Yoshihisa and Nakano, Takeshi and Mori, Tadashi}, journal = {Photochem. Photobiol. Sci.}, volume = {16}, issue = {4}, pages = {606--610}, year = {2017}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c6pp00438e}, url = {https://doi.org/10.1039/c6pp00438e}, dimensions = {true}, tab = {paper}, } -
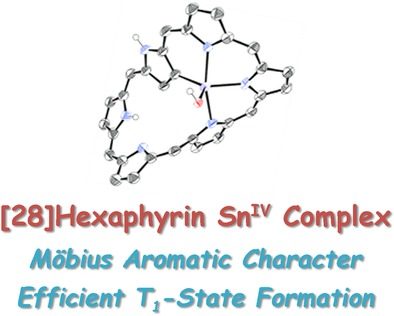 Möbius aromatic [28] hexaphyrin germanium (IV) and tin (IV) complexes: Efficient formation of triplet excited statesMondo Izawa, Taeyeon Kim, Shin-ichiro Ishida, Takayuki Tanaka, Tadashi Mori, Dongho Kim*, and Atsuhiro Osuka*Angew. Chem. Int. Ed., 2017, 129, 4040–4044.
Möbius aromatic [28] hexaphyrin germanium (IV) and tin (IV) complexes: Efficient formation of triplet excited statesMondo Izawa, Taeyeon Kim, Shin-ichiro Ishida, Takayuki Tanaka, Tadashi Mori, Dongho Kim*, and Atsuhiro Osuka*Angew. Chem. Int. Ed., 2017, 129, 4040–4044.[28]Hexaphyrin GeIV and SnIV complexes were synthesized in high yields by reactions of [28]hexaphyrin with GeCl4 or SnCl4 in the presence of triethylamine. Both complexes display distinct 28π Möbius aromatic character and possess a trigonal bipyramidal geometry at the central GeIV or SnIV atom. The equatorial hydroxy group of the GeIV complex was smoothly exchanged with neutral nucleophiles, such as phenol derivatives and thiophenol, with retention of configuration. In the SnIV complex, intersystem crossing to the T1 state is remarkably enhanced owing to the effective heavy-atom effect, thus allowing the formation of the T1 tate in high yield. The T1 states of the GeIV and SnVI complexes were found to be antiaromatic on the basis of the transient absorption features in line with the Baird rule.
@article{izawa2017mobius, title = {Möbius aromatic [28] hexaphyrin germanium (IV) and tin (IV) complexes: Efficient formation of triplet excited states}, author = {Izawa, Mondo and Kim, Taeyeon and Ishida, Shin-ichiro and Tanaka, Takayuki and Mori, Tadashi and Kim, Dongho and Osuka, Atsuhiro}, journal = {Angew. Chem. Int. Ed.}, volume = {129}, issue = {14}, pages = {4040--4044}, year = {2017}, publisher = {Wiley Online Library}, doi = {10.1002/anie.201700063}, url = {https://doi.org/10.1002/anie.201700063}, dimensions = {true}, tab = {paper}, } -
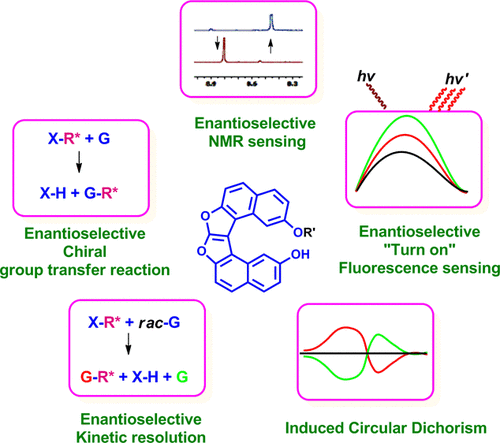 Sui generis helicene-based supramolecular chirogenic system: Enantioselective sensing, solvent control, and application in chiral group transfer reactionMohammed Hasan, Vaibhav N Khose, Tadashi Mori, Victor Borovkov*, and Anil V Karnik*ACS Omega, 2017, 2, 592–598.
Sui generis helicene-based supramolecular chirogenic system: Enantioselective sensing, solvent control, and application in chiral group transfer reactionMohammed Hasan, Vaibhav N Khose, Tadashi Mori, Victor Borovkov*, and Anil V Karnik*ACS Omega, 2017, 2, 592–598.A novel dioxa[6]helicene-based supramolecular chirogenic system (1) as a specific chiral recognition host for enantiopure trans-1,2-cyclohexanediamine (2) is reported. Host 1 with an inherent free phenolic group and a (1S)-camphanate chiral handle on the opposite terminal rings of the helicene chromophore acted as an efficient turn on fluorescent sensor for S,S-2 with an excellent enantioselective factor, α = KSS/KRR = 6.3 in benzene. This specific host–guest interaction phenomenon is found to be solvent-dependent, which leads to an enantioselective chiral (camphanate) group transfer to the diamine guest molecule. In the case of R,R-2, the de value is up to 68% even at room temperature. Intriguingly, the induced helicity in dioxa[6]helicene diol 6, upon supramolecular hydrogen-bonding interactions, is of opposite sense with positive helicity for S,S-2 and negative helicity for R,R-2, as shown by circular dichroism spectroscopy and in combination with theoretical calculations. This chiral supramolecular system is found to be an excellent host–guest pair for enantiomeric recognition of 2, based on their electronic and steric factors.
@article{hasan2017sui, title = {Sui generis helicene-based supramolecular chirogenic system: Enantioselective sensing, solvent control, and application in chiral group transfer reaction}, author = {Hasan, Mohammed and Khose, Vaibhav N and Mori, Tadashi and Borovkov, Victor and Karnik, Anil V}, journal = {ACS Omega}, volume = {2}, issue = {2}, pages = {592--598}, year = {2017}, publisher = {ACS Publications}, doi = {10.1021/acsomega.6b00522}, url = {https://doi.org/10.1021/acsomega.6b00522}, dimensions = {true}, tab = {paper}, } -
 Temperature Dynamics of Single Molecular Antifreeze ProteinRio Okada, Tatsuya Arai, Daichi Fukami, Yuhuku Matsushita, Jae-won Chang, Hiroshi Sekiguchi, Noboru Ohta, Tadashi Mori, Masaki Nishijima, Keisuke Miyazawa, Takeshi Fukuma, Keigo Ikezaki, Sakae Tsuda, and Yuji C SasakiBiophysical Journal, 2017, 112, 323a.
Temperature Dynamics of Single Molecular Antifreeze ProteinRio Okada, Tatsuya Arai, Daichi Fukami, Yuhuku Matsushita, Jae-won Chang, Hiroshi Sekiguchi, Noboru Ohta, Tadashi Mori, Masaki Nishijima, Keisuke Miyazawa, Takeshi Fukuma, Keigo Ikezaki, Sakae Tsuda, and Yuji C SasakiBiophysical Journal, 2017, 112, 323a.AntiFreeze Proteins (=AFPs) bind to a surface of ice crystals and inhibit their growth. Some living organisms; fishes, insects and fungus, at low-temperature environment have several different types of AFPs. AFPs protect their body from freezing damages. To clarify the antifreeze effect of lpAFP (isolated from longsnout poacher, a fish living in the sea of Okhotsk) at single molecular level, we performed the single molecular observation using diffracted X-ray tracking (=DXT). DXT is the method to observe single molecular motions using X-rays and gold nanocrystals as probe. Gold nanocrystals are labeled on AFPs, and when irradiated with X-rays, diffracted spots from gold nanocrystals can be observed. We can observe the motion of the AFPs by tracking these spots. We performed the DXT experiments at SPring-8 BL40XU, and the spatial and time resolutions are the order of milli-radian and micro-second. In this study, we used lpAFP isolated from longsnout poacher, and observed the temperature dependency of the lpAFP’s single molecular brownian motion. As a result, lpAFP’s motion increased at 5 °C. Next, to ensure the relationship between the adsorption property and the maximum brownian motion, we observed the temperature dependency of the adsorption affinity to AgI thin film as ice surface. As a result, the adsorption amount to AgI of lpAFP was the largest at 2°C. These two experiments suggest that there is a strong correlation between lpAFP’s molecular motion and adsorption property. We acquired the temperature dependency of lpAFP using other experiments; circular dichroism (CD), atomic force microscopy (AFM), dynamic light scattering (DLS), and small angle X-ray scattering (SAXS, performed at SPring-8 BL40B2). In poster session, we discuss the detail experimental procedure and results.
@article{okada2017temperature, title = {Temperature Dynamics of Single Molecular Antifreeze Protein}, author = {Okada, Rio and Arai, Tatsuya and Fukami, Daichi and Matsushita, Yuhuku and Chang, Jae-won and Sekiguchi, Hiroshi and Ohta, Noboru and Mori, Tadashi and Nishijima, Masaki and Miyazawa, Keisuke and Fukuma, Takeshi and Ikezaki, Keigo and Tsuda, Sakae and Sasaki, Yuji C}, journal = {Biophysical Journal}, volume = {112}, issue = {3}, pages = {323a}, year = {2017}, publisher = {Elsevier}, doi = {10.1016/j.bpj.2016.11.1751}, url = {https://doi.org/10.1016/j.bpj.2016.11.1751}, dimensions = {true}, tab = {paper}, } -
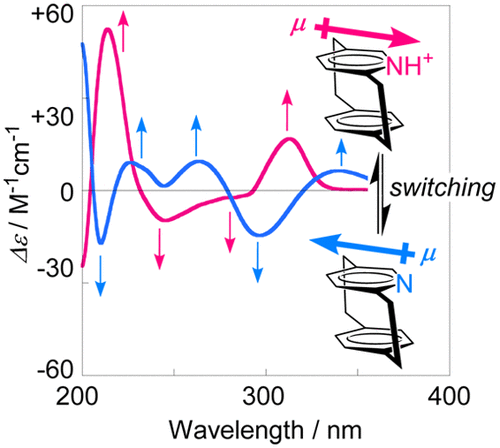 Protonation-induced sign inversion of the cotton effects of pyridinophanes. A combined experimental and theoretical studyAkinori Shimizu, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. A, 2017, 121, 977–985.
Protonation-induced sign inversion of the cotton effects of pyridinophanes. A combined experimental and theoretical studyAkinori Shimizu, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. A, 2017, 121, 977–985.The circular dichroisms (CDs) of planar chiral [2.2]- and [3.3]-pyridinophanes were investigated experimentally and theoretically. Strong multisignate Cotton effects, typical for cyclophane derivatives, were observed. The CD spectra of [2.2]- and [3.3]-paracyclophanes closely resembled in pattern each other, despite the much greater conformational variations in the latter. Upon protonation, both of the cyclophanes suffered dramatic CD spectral changes with accompanying complete sign inversion, which was attributed to the reversal of diploe moment of pyridinium versus pyridine moiety. This chiroptical property switching driven by protonation/deprotonation was temperature-dependent and hence applicable to thermal sensing. The protonated forms of pyridinophanes served as ideal model systems for studying the cation−π interactions and their effects on chiroptical properties. Thus, the molar CD (Δε) of the charge-transfer band of protonated [2.2]pyridinophane was 10-fold larger than that of protonated [3.3]pyridinophane, which exceeds the increased interplane electronic interactions assessed from the electronic coupling element values.
@article{shimizu2017protonation, title = {Protonation-induced sign inversion of the cotton effects of pyridinophanes. A combined experimental and theoretical study}, author = {Shimizu, Akinori and Inoue, Yoshihisa and Mori, Tadashi}, journal = {J. Phys. Chem. A}, volume = {121}, issue = {5}, pages = {977--985}, year = {2017}, publisher = {ACS Publications}, doi = {10.1021/acs.jpca.6b12287}, url = {https://doi.org/10.1021/acs.jpca.6b12287}, dimensions = {true}, tab = {paper}, } -
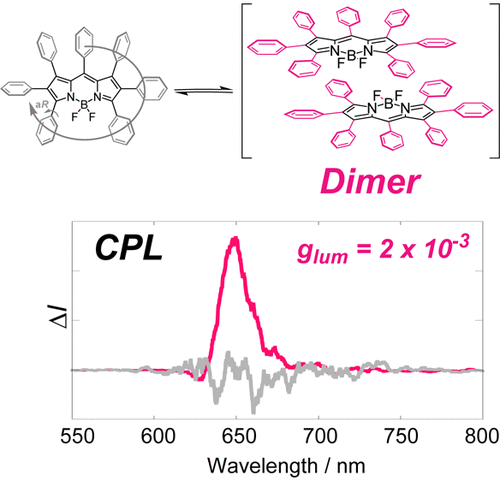 Propeller chirality of boron heptaaryldipyrromethene: unprecedented supramolecular dimerization and chiroptical propertiesMasataka Toyoda, Yoshitane Imai, and Tadashi Mori*J. Phys. Chem. Lett., 2017, 8, 42–48.
Propeller chirality of boron heptaaryldipyrromethene: unprecedented supramolecular dimerization and chiroptical propertiesMasataka Toyoda, Yoshitane Imai, and Tadashi Mori*J. Phys. Chem. Lett., 2017, 8, 42–48.Chiral boron dipyrromethenes (BPs) enjoy high fluorescence efficiency at visible to near-IR wavelength regions with a reasonable range of dissymmetry factors. Here, we demonstrate that the (quasi)propeller chirality, similarly to hexagonal propeller in hexaarylbenzene, can be effectively induced in heptaarylated BP. In addition, supramolecular dimer was formed at low temperatures in nonpolar solvent, which exhibits strong bisignate Cotton effects at BP transitions (the couplet amplitude A = 193 M–1 cm–1) in the circular dichroism (CD). Due to the bulky substituents on the propeller blades, but with void space around boron atoms, BP chromophores in the dimer are aligned in a head-to-tail manner with a small torsion (φ ≈ 15°), to avoid fluorescence quenching usually observed in H-type dimer of BPs, exhibiting strong circularly polarized luminescence (CPL) signals (glum = 2.0 × 10–3, Φlum = 0.45). Such supramolecular dimer formation would be viewed as an alternative approach for designing and developing novel chiroptical materials.
@article{toyoda2017propeller, title = {Propeller chirality of boron heptaaryldipyrromethene: unprecedented supramolecular dimerization and chiroptical properties}, author = {Toyoda, Masataka and Imai, Yoshitane and Mori, Tadashi}, journal = {J. Phys. Chem. Lett.}, volume = {8}, issue = {1}, pages = {42--48}, year = {2017}, publisher = {ACS Publications}, doi = {10.1021/acs.jpclett.6b02492}, url = {https://doi.org/10.1021/acs.jpclett.6b02492}, dimensions = {true}, tab = {paper}, } -
 Asymmetric Photochemical Synthesis Based on Selective Excitation of Charge-Transfer ComplexesTadashi Mori*J. Syn. Org. Chem., Jpn., 2017, 75, 144–152.
Asymmetric Photochemical Synthesis Based on Selective Excitation of Charge-Transfer ComplexesTadashi Mori*J. Syn. Org. Chem., Jpn., 2017, 75, 144–152.The use of photoreactions such as photoredox catalysts in asymmetric synthesis has been expansively recognized as expedient procedure over the past decade. In this paper, a potential application of photoreaction of Charge-Transfer (CT) complex for asymmetric synthesis is described. In chiral donor-acceptor systems, excitation wavelength, along with other environmental factors such as solvent polarity and temperature, plays more substantial role in determining the stereoselectivity of the photochemical outcomes. Accordingly, the excited CT complex produced upon the selective CT-band excitation behaves completely different from the conventional exciplex, which is formed by the direct excitation of donor or acceptor. Such unique wavelength effect, together with other entropic controls, may possibly come to be valuable means in asymmetric photochemical synthesis for the future.
@article{mori2017asymmetric, title = {Asymmetric Photochemical Synthesis Based on Selective Excitation of Charge-Transfer Complexes}, author = {Mori, Tadashi}, journal = {J. Syn. Org. Chem., Jpn.}, volume = {75}, number = {2}, pages = {144--152}, year = {2017}, doi = {10.5059/yukigoseikyokaishi.75.144}, url = {https://doi.org/10.5059/yukigoseikyokaishi.75.144}, dimensions = {true}, tab = {review}, } - Entropy Control of ReactionsTadashi MoriJ. Syn. Org. Chem., Jpn., 2017, 75, 160.
@article{mori2017entropy, title = {Entropy Control of Reactions}, author = {Mori, Tadashi}, journal = {J. Syn. Org. Chem., Jpn.}, volume = {75}, issue = {2}, pages = {160}, year = {2017}, doi = {10.5059/yukigoseikyokaishi.75.160}, url = {https://doi.org/10.5059/yukigoseikyokaishi.75.160}, dimensions = {true}, tab = {review} } - Chiral Photochemistry of Charge Transfer Complexes: Effect of Excitation WavelengthTadashi Mori*Symmetry, 2017, 28, 207–210.
@article{mori2017chiral, title = {Chiral Photochemistry of Charge Transfer Complexes: Effect of Excitation Wavelength}, author = {Mori, Tadashi}, journal = {Symmetry}, volume = {28}, issue = {2}, pages = {207--210}, year = {2017}, publisher = {Symmetry: Culture and Science} }