articles in 2016
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
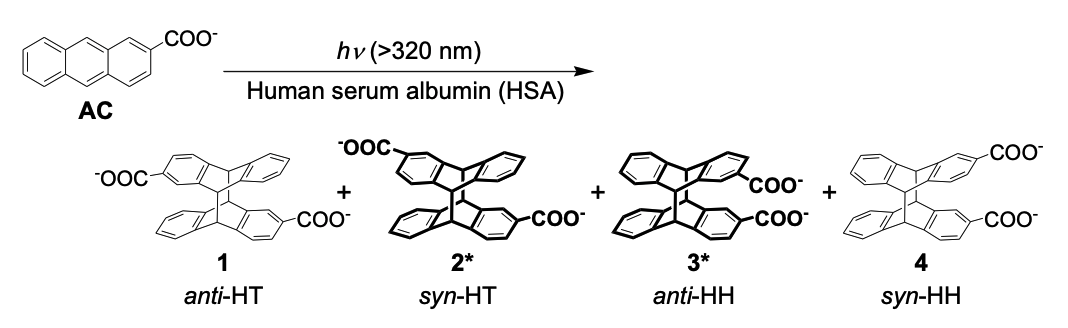 Highly enantiodifferentiating site of human serum albumin for mediating photocyclodimerization of 2-anthracenecarboxylate elucidated by site-specific inhibition/quenching with xenonMasaki Nishijima*, Tamara CS Pace, Cornelia Bohne, Tadashi Mori, Yoshihisa Inoue, and Takehiko WadaJ. Photochem. Photobiol. A: Chem., 2016, 331, 89–94.
Highly enantiodifferentiating site of human serum albumin for mediating photocyclodimerization of 2-anthracenecarboxylate elucidated by site-specific inhibition/quenching with xenonMasaki Nishijima*, Tamara CS Pace, Cornelia Bohne, Tadashi Mori, Yoshihisa Inoue, and Takehiko WadaJ. Photochem. Photobiol. A: Chem., 2016, 331, 89–94.Complementary to the previous assignment of the first, second, and fourth binding sites of human serum albumin (HSA) for 2-anthracenecarboxylate (AC) and the subsequent mediation of AC photocyclodimerization, the site-specific inhibition of the enantiodifferentiation by xenon allowed us to assign the remaining third and fifth AC-binding sites to subdomains IB and IIIB, respectively. This study reveals a clearer picture of the binding and photochirogenic behavior of HSA and further expands the scope of bio-supramolecular photochirogenesis.
@article{nishijima2016highly, title = {Highly enantiodifferentiating site of human serum albumin for mediating photocyclodimerization of 2-anthracenecarboxylate elucidated by site-specific inhibition/quenching with xenon}, author = {Nishijima, Masaki and Pace, Tamara CS and Bohne, Cornelia and Mori, Tadashi and Inoue, Yoshihisa and Wada, Takehiko}, journal = {J. Photochem. Photobiol. A: Chem.}, volume = {331}, pages = {89--94}, year = {2016}, publisher = {Elsevier}, doi = {10.1016/j.jphotochem.2015.12.019}, url = {https://doi.org/10.1016/j.jphotochem.2015.12.019}, dimensions = {true}, tab = {paper}, } -
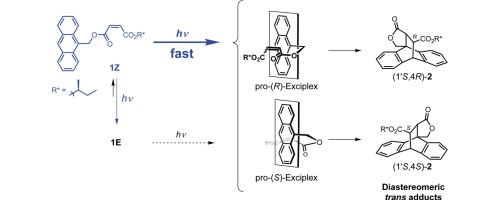 Enhanced asymmetric photocycloaddition of anthracene tethered to maleate versus fumarate through non-fluorescent exciplex intermediateMakoto Ichikawa, Yoshihisa Inoue, and Tadashi Mori*J. Photochem. Photobiol. A: Chem., 2016, 331, 102–109.
Enhanced asymmetric photocycloaddition of anthracene tethered to maleate versus fumarate through non-fluorescent exciplex intermediateMakoto Ichikawa, Yoshihisa Inoue, and Tadashi Mori*J. Photochem. Photobiol. A: Chem., 2016, 331, 102–109.The asymmetric photocycloaddition of anthracene-tethered maleate with a small (S)-1-methylpropyl auxiliary at the peripheral position (1Z) underwent much faster than that of the corresponding fumarate (1E). The absolute configuration of the newly created chiral center in the major product was ambiguously assigned as (4S) by X-ray crystallographic as well as CD spectral studies. The facile E–Z photoisomerization was simultaneously observed for both 1Z and 1E, again much faster for 1Z. The concurrent electron transfer works as a quenching process and the fumarate acted as a better quencher than maleate in both intramolecular and intermolecular systems. The fluorescence quenching and quantum yield investigations also supported such facile electron transfer from excited-state anthracene to acceptor in the singlet manifold. Observed diastereoselectivities in the photocycloaddition of 1Z and 1E were moderate but substantial (5–12% de), given the fact that the chiral auxiliary is introduced at the remote position from the reaction center (4-bond separation). The non-fluorescent exciplex, probably in a triplet manifold derived from the singlet exciplex, plays a crucial role in this efficient cyclization process, affording a biradical intermediate, that can either dissociate or undergo second bond formation to afford a diastereomeric trans adduct 2. The diastereoselectivity was higher in less polar solvents, suggesting the polar nature of the exciplex.
@article{ichikawa2016enhanced, title = {Enhanced asymmetric photocycloaddition of anthracene tethered to maleate versus fumarate through non-fluorescent exciplex intermediate}, author = {Ichikawa, Makoto and Inoue, Yoshihisa and Mori, Tadashi}, journal = {J. Photochem. Photobiol. A: Chem.}, volume = {331}, pages = {102--109}, year = {2016}, publisher = {Elsevier}, doi = {10.1016/j.jphotochem.2015.09.001}, url = {https://doi.org/10.1016/j.jphotochem.2015.09.001}, dimensions = {true}, tab = {paper}, } -
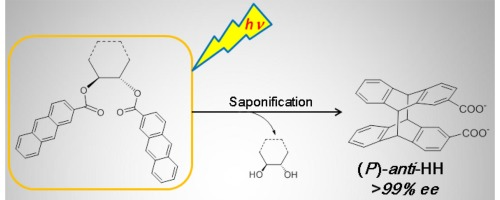 Critical control by scaffold flexibility achieved in diastereodifferentiating photocyclodimerization of 2-anthracenecarboxylateGaku Fukuhara*, Kazuhiro Iida, Tadashi Mori, and Yoshihisa InoueJ. Photochem. Photobiol. A: Chem., 2016, 331, 76–83.
Critical control by scaffold flexibility achieved in diastereodifferentiating photocyclodimerization of 2-anthracenecarboxylateGaku Fukuhara*, Kazuhiro Iida, Tadashi Mori, and Yoshihisa InoueJ. Photochem. Photobiol. A: Chem., 2016, 331, 76–83.By progressively increasing the flexibility of chiral vicinal diol scaffold (from rigid cyclic tetrasaccharide to flexible 2,3-butanediol via glucose and trans-1,2-cyclohexandiol) in the diastereodifferentiating photocyclodimerization to head-to-head (HH) dimers of 2-anthracenecarboxylate on the scaffold, the anti/syn preference was dramatically inverted from 42:1 to 1:12, while the enantiomeric excess of the chiral anti-HH dimer was consistently kept high at >99% due to the excited-state dynamics that strongly disfavors the si–si enantiotopic face attack against the antipodal re–re face attack, exclusively affording the (P)-enantiomer.
@article{fukuhara2016critical, title = {Critical control by scaffold flexibility achieved in diastereodifferentiating photocyclodimerization of 2-anthracenecarboxylate}, author = {Fukuhara, Gaku and Iida, Kazuhiro and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Photochem. Photobiol. A: Chem.}, volume = {331}, pages = {76--83}, year = {2016}, publisher = {Elsevier}, doi = {10.1016/j.jphotochem.2016.01.016}, url = {https://doi.org/10.1016/j.jphotochem.2016.01.016}, dimensions = {true}, tab = {paper}, } -
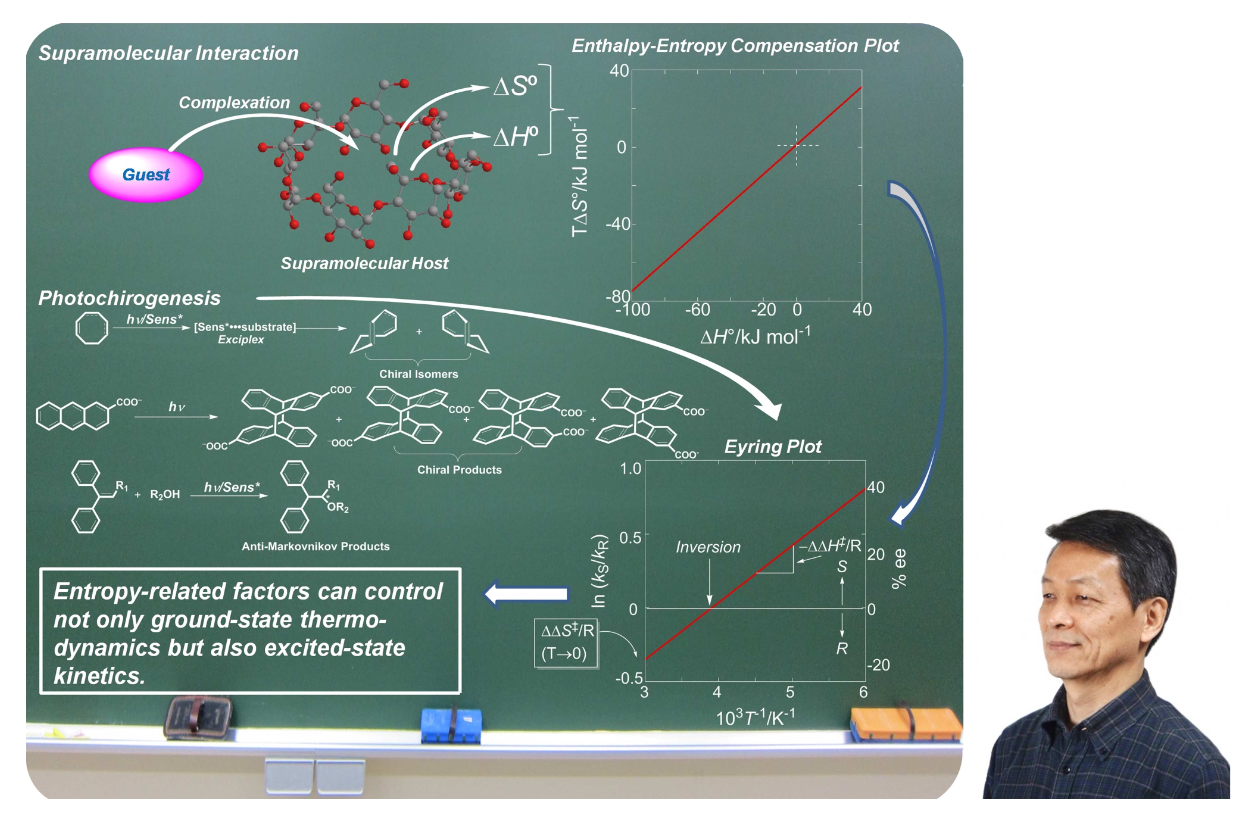 Yoshihisa Inoue—A researcher’s quest for photochirogenesisTadashi Mori*, Gaku Fukuhara, and Takehiko WadaJ. Photochem. Photobiol. A: Chem., 2016, 331, 2–7.
Yoshihisa Inoue—A researcher’s quest for photochirogenesisTadashi Mori*, Gaku Fukuhara, and Takehiko WadaJ. Photochem. Photobiol. A: Chem., 2016, 331, 2–7.This issue of Journal of Photochemistry and Photobiology A: Chemistry is dedicated to Prof. Yoshihisa Inoue, a man of “photochirogenesis”, and his enthusiastic attitude toward chemistry. We feel highly privileged and deeply honored that we have been serving as guest editors for this special issue, honoring his retirement from Osaka University in 2015 and continued impact on supramolecular chemistry and photochemistry.
@article{mori2016yoshihisa, title = {Yoshihisa Inoue—A researcher’s quest for photochirogenesis}, author = {Mori, Tadashi and Fukuhara, Gaku and Wada, Takehiko}, journal = {J. Photochem. Photobiol. A: Chem.}, volume = {331}, pages = {2--7}, year = {2016}, publisher = {Elsevier}, doi = {10.1016/j.jphotochem.2016.08.006}, url = {https://doi.org/10.1016/j.jphotochem.2016.08.006}, dimensions = {true}, tab = {paper}, } -
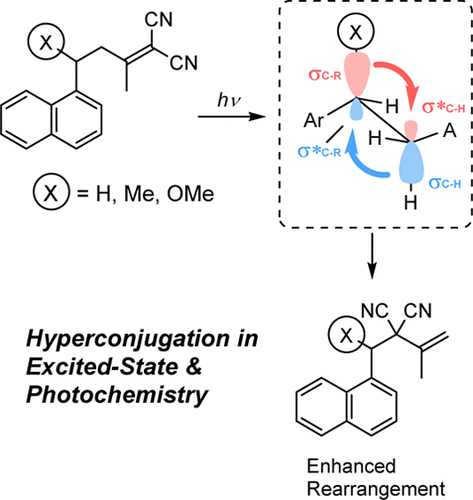 Orbital Control of Photochemical Rearrangement of 4-Aryl-1, 1-dicyano-1-butenes through the Hyperconjugative Substitution on the Linker ChainNobuo Matsuki, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. Lett., 2016, 7, 4957–4961.
Orbital Control of Photochemical Rearrangement of 4-Aryl-1, 1-dicyano-1-butenes through the Hyperconjugative Substitution on the Linker ChainNobuo Matsuki, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. Lett., 2016, 7, 4957–4961.Hyperconjugative interaction was demonstrated to play a vital role in the photochemistry of 4-aryl-1,1-dicyano-1-butenes. Thus a simple substituent on the benzylic position effectively induced a new photoreactivity to afford an allylic rearrangement product that is not obtained for the parent substrate. The natural bond orbital analysis was employed to reveal the enhanced relative contributions of hyperconjugation in the excited state, which dramatically alter the photochemical outcomes not only by reducing the strength of the allylic/benzylic bond but more crucially by affecting the conformer distribution.
@article{matsuki2016orbital, title = {Orbital Control of Photochemical Rearrangement of 4-Aryl-1, 1-dicyano-1-butenes through the Hyperconjugative Substitution on the Linker Chain}, author = {Matsuki, Nobuo and Inoue, Yoshihisa and Mori, Tadashi}, journal = {J. Phys. Chem. Lett.}, volume = {7}, issue = {24}, pages = {4957--4961}, year = {2016}, publisher = {ACS Publications}, doi = {10.1021/acs.jpclett.6b02632}, url = {https://doi.org/10.1021/acs.jpclett.6b02632}, dimensions = {true}, tab = {paper}, } -
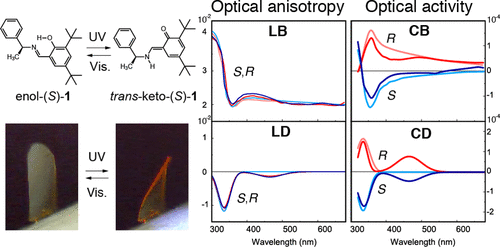 Optical activity and optical anisotropy in photomechanical crystals of chiral salicylidenephenylethylaminesAkifumi Takanabe, Masahito Tanaka, Kohei Johmoto, Hidehiro Uekusa, Tadashi Mori, Hideko Koshima*, and Toru Asahi*J. Am. Chem. Soc., 2016, 138, 15066–15077.
Optical activity and optical anisotropy in photomechanical crystals of chiral salicylidenephenylethylaminesAkifumi Takanabe, Masahito Tanaka, Kohei Johmoto, Hidehiro Uekusa, Tadashi Mori, Hideko Koshima*, and Toru Asahi*J. Am. Chem. Soc., 2016, 138, 15066–15077.Introducing chirality into photomechanical crystals is beneficial for the diversification of mechanical motion. Measurement of the chiroptical and optical anisotropic properties of chiral crystals is indispensable for evaluating photomechanical crystals. The platelike crystals of S- and R-enantiomers of photochromic N-3,5-di-tert-butylsalicylidene-1-phenylethylamine in enol form (enol-(S)-1 and enol-(R)-1) caused bending motion with twisting upon ultraviolet (UV) light irradiation, due to shrinkage along the length and width directions of the irradiated surface, based on the optimized crystal structure of the photoisomerized trans-keto-(S)-1. By employing the generalized high-accuracy universal polarimeter (G-HAUP), optical anisotropic (linear birefringence, LB; linear dichroism, LD) as well as chiroptical (circular birefringence, CB; circular dichroism, CD) spectra of both the enantiomeric crystals on the (001) face were simultaneously measured before and under continuous UV irradiation. The LD peak was observed at 330 nm in the negative sign, derived from the π–π* transition of the intramolecularly hydrogen-bonded salicylidenimino moiety. The CD spectra of the S and R crystals revealed the negative and positive Cotton effect at 330 nm, respectively, and new peaks appeared at 460 nm under UV light irradiation due to photoisomerization to the S and Rtrans-keto isomers at around 10% conversion. The CB and CD spectra evaluated by the HAUP measurement were opposite to those measured in the hexane solution, as well as those simulated by quantum chemical calculation. The dissymmetry parameter, g, of the enol-(S)-1 crystal along the c axis (0.013) was approximately 10 times larger than the g values in the solution (0.0010) and by calculation (0.0016).
@article{takanabe2016optical, title = {Optical activity and optical anisotropy in photomechanical crystals of chiral salicylidenephenylethylamines}, author = {Takanabe, Akifumi and Tanaka, Masahito and Johmoto, Kohei and Uekusa, Hidehiro and Mori, Tadashi and Koshima, Hideko and Asahi, Toru}, journal = {J. Am. Chem. Soc.}, volume = {138}, issue = {45}, pages = {15066--15077}, year = {2016}, publisher = {ACS Publications}, doi = {10.1021/jacs.6b09633}, url = {https://doi.org/10.1021/jacs.6b09633}, dimensions = {true}, tab = {paper}, } -
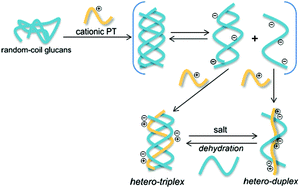 Electrostatically promoted dynamic hybridization of glucans with cationic polythiopheneGaku Fukuhara*, Mami Imai, Denis Fuentealba, Yuki Ishida, Hiroki Kurohara, Cheng Yang, Tadashi Mori, Hiroshi Uyama, Cornelia Bohne, and Yoshihisa InoueOrg. Biomol. Chem., 2016, 14, 9741–9750.
Electrostatically promoted dynamic hybridization of glucans with cationic polythiopheneGaku Fukuhara*, Mami Imai, Denis Fuentealba, Yuki Ishida, Hiroki Kurohara, Cheng Yang, Tadashi Mori, Hiroshi Uyama, Cornelia Bohne, and Yoshihisa InoueOrg. Biomol. Chem., 2016, 14, 9741–9750.Hybridizing natural macromolecules with synthetic polymers is an efficient general method for constructing sophisticated supramolecular architectures. To comprehensively elucidate the controversial hybridization mechanism of glucans with synthetic polymers, the hybridization behaviors of triple-stranded curdlan (Cur) and schizophyllan (SPG) with cationic polythiophene (PyPT) were investigated in aqueous DMSO solutions by using UV-vis, circular dichroism (CD), fluorescence, fluorescence excitation, and NMR spectroscopy methods, as well as theoretical calculations, dynamic light scattering, and zeta potential measurements. Upon mixing with glucan, a hetero-triplex formed, which was dynamic and greatly accelerated by heating and by adding a base or a salt. The hetero-triplex disassembled into a hetero-duplex in highly basic solutions. Thus, polycationic polymers, such as PyPT, are expected to serve as a versatile tool for unzipping glucan homo-triplexes and promoting subsequent hybridization in aqueous solution, while the detailed mechanism elucidated in the present study contributes to the rational design of hybridization partners.
@article{fukuhara2016electrostatically, title = {Electrostatically promoted dynamic hybridization of glucans with cationic polythiophene}, author = {Fukuhara, Gaku and Imai, Mami and Fuentealba, Denis and Ishida, Yuki and Kurohara, Hiroki and Yang, Cheng and Mori, Tadashi and Uyama, Hiroshi and Bohne, Cornelia and Inoue, Yoshihisa}, journal = {Org. Biomol. Chem.}, volume = {14}, number = {41}, pages = {9741--9750}, year = {2016}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c6ob01353h}, url = {https://doi.org/10.1039/c6ob01353h}, dimensions = {true}, tab = {paper}, } -
 Supramolecular photochirogenesis with a higher-order complex: highly accelerated exclusively head-to-head photocyclodimerization of 2-anthracenecarboxylic acid via 2: 2 complexation with prolinolYuko Kawanami, Shin-ya Katsumata, Masaki Nishijima, Gaku Fukuhara, Kaori Asano, Takeyuki Suzuki, Cheng Yang, Asao Nakamura, Tadashi Mori, and Yoshihisa Inoue*J. Am. Chem. Soc., 2016, 138, 12187–12201.
Supramolecular photochirogenesis with a higher-order complex: highly accelerated exclusively head-to-head photocyclodimerization of 2-anthracenecarboxylic acid via 2: 2 complexation with prolinolYuko Kawanami, Shin-ya Katsumata, Masaki Nishijima, Gaku Fukuhara, Kaori Asano, Takeyuki Suzuki, Cheng Yang, Asao Nakamura, Tadashi Mori, and Yoshihisa Inoue*J. Am. Chem. Soc., 2016, 138, 12187–12201.An unprecedented 2:2 complex was shown to intervene in the enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid (A) mediated by a hydrogen-bonding template l-prolinol (P) to accelerate the formation of chiral anti-head-to-head and achiral syn-head-to-head cyclodimers in >99% combined yield with enhanced enantioselectivities of up to 72% ee for the former. The supramolecular complexation and photochirogenic behaviors, as well as the plausible structures, of intervening Am·Pn complexes (m, n = 1 or 2) were elucidated by combined theoretical and experimental spectroscopic, photophysical, and photochemical studies. Furthermore, the photochemical chiral amplification was achieved for the first time by utilizing the preferential 2:2 complexation of A with homochiral P to give normalized product enantioselectivities higher than those of the template used. The present strategy based on the higher-order hydrogen-bonding motif, which is potentially applicable to a variety of carboxylic acids and β-aminoalcohols, is not only conceptually new and expandable to other (photo)chirogenic and sensing systems but also may serve as a versatile tool for achieving photochemical asymmetric amplification and constructing chiral functional supramolecular architectures.
@article{kawanami2016supramolecular, title = {Supramolecular photochirogenesis with a higher-order complex: highly accelerated exclusively head-to-head photocyclodimerization of 2-anthracenecarboxylic acid via 2: 2 complexation with prolinol}, author = {Kawanami, Yuko and Katsumata, Shin-ya and Nishijima, Masaki and Fukuhara, Gaku and Asano, Kaori and Suzuki, Takeyuki and Yang, Cheng and Nakamura, Asao and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Am. Chem. Soc.}, volume = {138}, number = {37}, pages = {12187--12201}, year = {2016}, publisher = {ACS Publications}, doi = {10.1021/jacs.6b05598}, url = {https://doi.org/10.1021/jacs.6b05598}, dimensions = {true}, tab = {paper}, } -
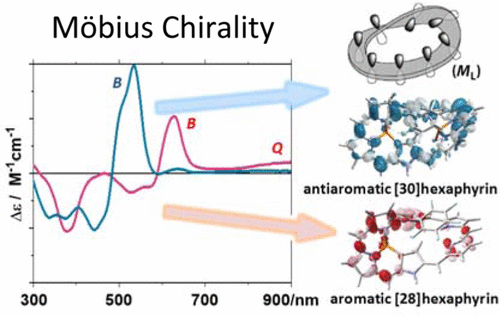 Combined Experimental and Theoretical Investigations on Optical Activities of Möbius Aromatic and Möbius Antiaromatic Hexaphyrin Phosphorus ComplexesTadashi Mori*, Takayuki Tanaka, Tomohiro Higashino, Kota Yoshida, and Atsuhiro OsukaJ. Phys. Chem. A, 2016, 120, 4241–4248.
Combined Experimental and Theoretical Investigations on Optical Activities of Möbius Aromatic and Möbius Antiaromatic Hexaphyrin Phosphorus ComplexesTadashi Mori*, Takayuki Tanaka, Tomohiro Higashino, Kota Yoshida, and Atsuhiro OsukaJ. Phys. Chem. A, 2016, 120, 4241–4248.Intrinsically chiral Möbius aromatic [28]hexaphyrin monophosphorus(V) and Möbius antiaromatic [30]hexaphyrin bisphosphorus(V) complexes have been optically resolved and their absolute configurations (ACs) were determined by combined experimental and theoretical investigations on their circular dichroisms (CDs). First elutes in chiral HPLC exhibited strong positive Cotton effects (CEs) at the B-band, characteristic for the ML configurations in their Möbius strips. Weak CEs at the Q-band, if attainable, complemented their AC assignment. The whole CD pattern and intensity were well reproduced by time-dependent approximate coupled cluster theory using model systems that omit five outward meso-aryl substituents (inward-meso-retained model), providing a solid basis for AC assignment. The cost efficient TD-DFT method with appropriate functionals for fully substituted (nontruncated) complexes well reproduced CEs around the B-band (but less satisfactory at the Q-band), also allows the rapid AC estimation for their Möbius strips. Observed difference in CDs between aromatic and antiaromatic hexaphyrins were better interpreted by their shifts in energy levels and altered interactions of relevant molecular orbitals, rather than small differences in Möbius geometries nor aromatic/antiaromatic character, despite the correlations recently claimed in planar π-systems.
@article{mori2016combined, title = {Combined Experimental and Theoretical Investigations on Optical Activities of Möbius Aromatic and Möbius Antiaromatic Hexaphyrin Phosphorus Complexes}, author = {Mori, Tadashi and Tanaka, Takayuki and Higashino, Tomohiro and Yoshida, Kota and Osuka, Atsuhiro}, journal = {J. Phys. Chem. A}, volume = {120}, number = {24}, pages = {4241--4248}, year = {2016}, publisher = {ACS Publications}, doi = {10.1021/acs.jpca.6b03978}, url = {https://doi.org/10.1021/acs.jpca.6b03978}, dimensions = {true}, tab = {paper}, } -
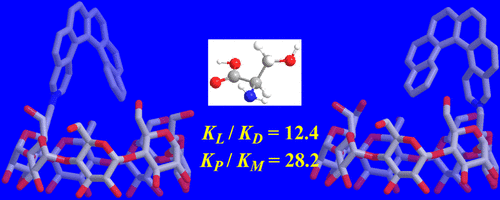 Inherently chiral azonia [6] helicene-modified β-cyclodextrin: Synthesis, characterization, and chirality sensing of underivatized amino acids in waterQinfei Huang, Liangwei Jiang, Wenting Liang, Jianchang Gui, Dingguo Xu, Wanhua Wu, Yoshito Nakai, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cheng Yang*J. Org. Chem., 2016, 81, 3430–3434.
Inherently chiral azonia [6] helicene-modified β-cyclodextrin: Synthesis, characterization, and chirality sensing of underivatized amino acids in waterQinfei Huang, Liangwei Jiang, Wenting Liang, Jianchang Gui, Dingguo Xu, Wanhua Wu, Yoshito Nakai, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cheng Yang*J. Org. Chem., 2016, 81, 3430–3434.The (P)- and (M)-3-azonia[6]helicenyl β-cyclodextrins exhibit l/d selectivities of up to 12.4 and P/M preferences of up to 28.2 upon complexation with underivatized proteinogenic amino acids in aqueous solution at pH 7.3.
@article{huang2016inherently, title = {Inherently chiral azonia [6] helicene-modified $\beta$-cyclodextrin: Synthesis, characterization, and chirality sensing of underivatized amino acids in water}, author = {Huang, Qinfei and Jiang, Liangwei and Liang, Wenting and Gui, Jianchang and Xu, Dingguo and Wu, Wanhua and Nakai, Yoshito and Nishijima, Masaki and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa and Yang, Cheng}, journal = {J. Org. Chem.}, volume = {81}, number = {8}, pages = {3430--3434}, year = {2016}, publisher = {ACS Publications}, doi = {10.1021/acs.joc.6b00130}, url = {https://doi.org/10.1021/acs.joc.6b00130}, dimensions = {true}, tab = {paper}, } -
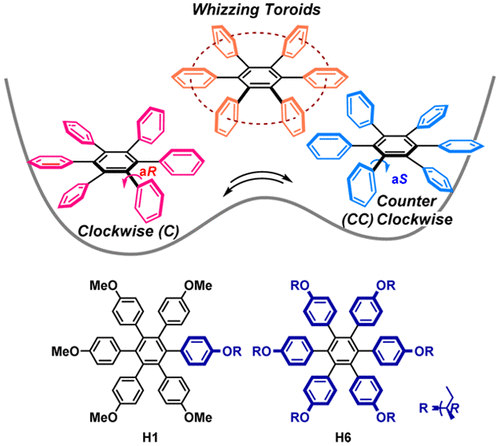 Toroidal interaction and propeller chirality of hexaarylbenzenes. Dynamic domino inversion revealed by combined experimental and theoretical circular dichroism studiesTomoyo Kosaka, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. Lett., 2016, 7, 783–788.
Toroidal interaction and propeller chirality of hexaarylbenzenes. Dynamic domino inversion revealed by combined experimental and theoretical circular dichroism studiesTomoyo Kosaka, Yoshihisa Inoue, and Tadashi Mori*J. Phys. Chem. Lett., 2016, 7, 783–788.Hexaarylbenzenes (HABs) have greatly attracted much attention due to their unique propeller-shaped structure and potential application in materials science, such as liquid crystals, molecular capsules/rotors, redox materials, nonlinear optical materials, as well as molecular wires. Less attention has however been paid to their propeller chirality. By introducing small point-chiral group(s) at the periphery of HABs, propeller chirality was effectively induced, provoking strong Cotton effects in the circular dichroism (CD) spectrum. Temperature and solvent polarity manipulate the dynamics of propeller inversion in solution. As such, whizzing toroids become more substantial in polar solvents and at an elevated temperature, where radial aromatic rings (propeller blades) prefer orthogonal alignment against the central benzene ring (C6 core), maximizing toroidal interactions.
@article{kosaka2016toroidal, title = {Toroidal interaction and propeller chirality of hexaarylbenzenes. Dynamic domino inversion revealed by combined experimental and theoretical circular dichroism studies}, author = {Kosaka, Tomoyo and Inoue, Yoshihisa and Mori, Tadashi}, journal = {J. Phys. Chem. Lett.}, volume = {7}, number = {5}, pages = {783--788}, year = {2016}, publisher = {ACS Publications}, doi = {10.1021/acs.jpclett.6b00179}, url = {https://doi.org/10.1021/acs.jpclett.6b00179}, dimensions = {true}, tab = {paper}, } -
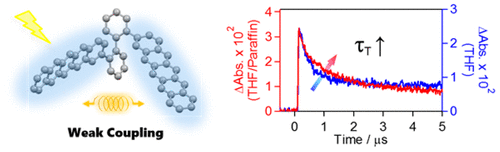 Long-lived triplet excited states of bent-shaped pentacene dimers by intramolecular singlet fissionTakao Sakuma, Hayato Sakai, Yasuyuki Araki, Tadashi Mori, Takehiko Wada, Nikolai V Tkachenko*, and Taku Hasobe*J. Phys. Chem. A, 2016, 120, 1867–1875.
Long-lived triplet excited states of bent-shaped pentacene dimers by intramolecular singlet fissionTakao Sakuma, Hayato Sakai, Yasuyuki Araki, Tadashi Mori, Takehiko Wada, Nikolai V Tkachenko*, and Taku Hasobe*J. Phys. Chem. A, 2016, 120, 1867–1875.Intramolecular singlet fission (ISF) is a promising photophysical process to construct more efficient light energy conversion systems as one excited singlet state converts into two excited triplet states. Herein we synthesized and evaluated bent-shaped pentacene dimers as a prototype of ISF to reveal intrinsic characters of triplet states (e.g., lifetimes of triplet excited states). In this study, meta-phenylene-bridged TIPS-pentacene dimer (PcD-3Ph) and 2,2′-bipheynyl bridged TIPS-pentacene dimer (PcD-Biph) were newly synthesized as bent-shaped dimers. In the steady-state spectroscopy, absorption and emission bands of these dimers were fully characterized, suggesting the appropriate degree of electronic coupling between pentacene moieties in these dimers. In addition, the electrochemical measurements were also performed to check the electronic interaction between two pentacene moieties. Whereas the successive two oxidation peaks owing to the delocalization were observed in a directly linked-pentacene dimer (PcD) by a single bond, the cyclic voltammograms in PcD-Biph and PcD-3Ph implied the weaker interaction compared to that of p-phenylene-bridged TIPS-pentacene dimer (PcD-4Ph) and PcD. The femtosecond and nanosecond transient absorption spectra clearly revealed the slower ISF process in bent-shaped pentacene dimers (PcD-Biph and PcD-3Ph), more notably, the slower relaxation of the excited triplet states in PcD-Biph and PcD-3Ph. Namely, the quantum yields of triplet states (ΦT) by ISF approximately remain constant (ca. 180–200%) in all dimer systems, whereas the lifetimes of the triplet excited states became much longer (up to 360 ns) in PcD-Biph as compared to PcD-4Ph (15 ns). Additionally, the lifetimes of the corresponding triplet states in PcD-Biph and PcD-3Ph were sufficiently affected by solvent viscosity. In particular, the lifetimes of PcD-Biph triplet state in THF/paraffin (1.0 μs) increased up to approximately three times as compared to that in THF (360 ns), whereas those of PcD-4Ph were quite similar in both solvent.
@article{sakuma2016long, title = {Long-lived triplet excited states of bent-shaped pentacene dimers by intramolecular singlet fission}, author = {Sakuma, Takao and Sakai, Hayato and Araki, Yasuyuki and Mori, Tadashi and Wada, Takehiko and Tkachenko, Nikolai V and Hasobe, Taku}, journal = {J. Phys. Chem. A}, volume = {120}, number = {11}, pages = {1867--1875}, year = {2016}, publisher = {ACS Publications}, doi = {10.1021/acs.jpca.6b00988}, url = {https://doi.org/10.1021/acs.jpca.6b00988}, dimensions = {true}, tab = {paper}, } -
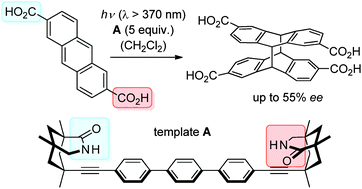 Enantioselective [4+4] photodimerization of anthracene-2,6-dicarboxylic acid mediated by a C2-symmetric chiral templateMark M Maturi, Gaku Fukuhara, Koichiro Tanaka, Yuko Kawanami, Tadashi Mori, Yoshihisa Inoue*, and Thorsten BachChem. Commun., 2016, 52, 1032–1035.
Enantioselective [4+4] photodimerization of anthracene-2,6-dicarboxylic acid mediated by a C2-symmetric chiral templateMark M Maturi, Gaku Fukuhara, Koichiro Tanaka, Yuko Kawanami, Tadashi Mori, Yoshihisa Inoue*, and Thorsten BachChem. Commun., 2016, 52, 1032–1035.A chiral template was constructed from 7-ethynyl-1,5,7-trimethyl-3-azabicyclo[3.3.1]nonan-2-one by Sonogashira cross-coupling with 4,4′′-diiodoterphenyl and was shown to bind the title compound strongly by hydrogen bonding resulting in enantioselectivities of up to 55% enantiomeric excess (ee) in the [4+4] anthracene photodimerization.
@article{maturi2016enantioselective, title = {Enantioselective [4+4] photodimerization of anthracene-2,6-dicarboxylic acid mediated by a C2-symmetric chiral template}, author = {Maturi, Mark M and Fukuhara, Gaku and Tanaka, Koichiro and Kawanami, Yuko and Mori, Tadashi and Inoue, Yoshihisa and Bach, Thorsten}, journal = {Chem. Commun.}, volume = {52}, number = {5}, pages = {1032--1035}, year = {2016}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c5cc09107a}, url = {https://doi.org/10.1039/c5cc09107a}, dimensions = {true}, tab = {paper}, } -
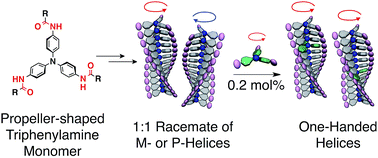 Dynamic propeller conformation for the unprecedentedly high degree of chiral amplification of supramolecular helicesTaehoon Kim, Tadashi Mori, Takuzo Aida, and Daigo Miyajima*Chem. Sci., 2016, 7, 6689–6694.
Dynamic propeller conformation for the unprecedentedly high degree of chiral amplification of supramolecular helicesTaehoon Kim, Tadashi Mori, Takuzo Aida, and Daigo Miyajima*Chem. Sci., 2016, 7, 6689–6694.An unprecedentedly high degree of chiral amplification of supramolecular helices in a sergeants and soldiers system was realized using a propeller-shaped molecule, triphenylamine (TPA), as the monomer. One sergeant controlled the handedness of 500 soldiers in supramolecular helices. We further demonstrated that a TPA derivative could switch its role from sergeant to soldier and vice versa depending on its partners. These achievements could be realized using the dynamic propeller conformation of TPA and provide new insights into supramolecular assemblies and the supramolecular chiral amplification of helices.
@article{kim2016dynamic, title = {Dynamic propeller conformation for the unprecedentedly high degree of chiral amplification of supramolecular helices}, author = {Kim, Taehoon and Mori, Tadashi and Aida, Takuzo and Miyajima, Daigo}, journal = {Chem. Sci.}, volume = {7}, number = {11}, pages = {6689--6694}, year = {2016}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c6sc02814d}, url = {https://doi.org/10.1039/c6sc02814d}, dimensions = {true}, tab = {paper}, }