articles in 2015
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
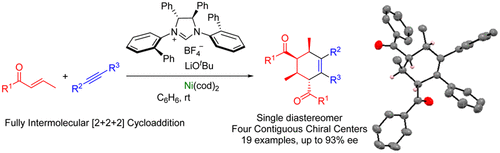 Nickel (0)/N-heterocyclic carbene-catalyzed asymmetric [2+ 2+ 2] cycloaddition of two enones and an alkyne: Access to cyclohexenes with four contiguous stereogenic centersRavindra Kumar, Hiromu Tokura, Akira Nishimura, Tadashi Mori, Yoichi Hoshimoto, Masato Ohashi, and Sensuke Ogoshi*Org. Lett., 2015, 17, 6018–6021.
Nickel (0)/N-heterocyclic carbene-catalyzed asymmetric [2+ 2+ 2] cycloaddition of two enones and an alkyne: Access to cyclohexenes with four contiguous stereogenic centersRavindra Kumar, Hiromu Tokura, Akira Nishimura, Tadashi Mori, Yoichi Hoshimoto, Masato Ohashi, and Sensuke Ogoshi*Org. Lett., 2015, 17, 6018–6021.A nickel(0)/chiral N-heterocyclic carbene (NHC)-catalyzed fully intermolecular, enantioselective [2 + 2 + 2] cycloaddition of two enones and an alkyne has been developed to access enantioenriched cyclohexenes. A single diastereomer was obtained with a successive generation of four contiguous stereogenic centers. The absolute configuration of cyclohexene derivative 3aa was determined by X-ray diffraction and circular dichroism (CD) spectral studies.
@article{kumar2015nickel, title = {Nickel (0)/N-heterocyclic carbene-catalyzed asymmetric [2+ 2+ 2] cycloaddition of two enones and an alkyne: Access to cyclohexenes with four contiguous stereogenic centers}, author = {Kumar, Ravindra and Tokura, Hiromu and Nishimura, Akira and Mori, Tadashi and Hoshimoto, Yoichi and Ohashi, Masato and Ogoshi, Sensuke}, journal = {Org. Lett.}, volume = {17}, number = {24}, pages = {6018--6021}, year = {2015}, publisher = {ACS Publications}, doi = {10.1021/acs.orglett.5b02983}, url = {https://doi.org/10.1021/acs.orglett.5b02983}, dimensions = {true}, tab = {paper}, } -
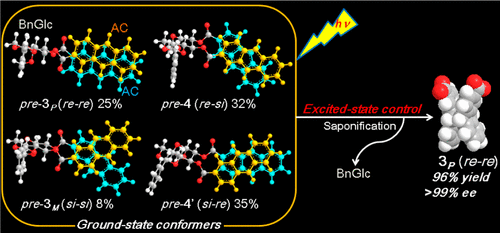 Excited-state dynamics achieved ultimate stereocontrol of photocyclodimerization of anthracenecarboxylates on a glucose scaffoldGaku Fukuhara*, Kazuhiro Iida, Yuko Kawanami, Hidekazu Tanaka, Tadashi Mori, and Yoshihisa Inoue*J. Am. Chem. Soc., 2015, 137, 15007–15014.
Excited-state dynamics achieved ultimate stereocontrol of photocyclodimerization of anthracenecarboxylates on a glucose scaffoldGaku Fukuhara*, Kazuhiro Iida, Yuko Kawanami, Hidekazu Tanaka, Tadashi Mori, and Yoshihisa Inoue*J. Am. Chem. Soc., 2015, 137, 15007–15014.Near-perfect stereoselectivity was attained in the diastereodifferentiating [4 + 4] photocyclodimerization of 2-anthracenecarboxylates tethered to a glucose scaffold not by thermodynamically tuning the conformer equilibrium in the ground state but by kinetically controlling the conformer dynamics and reactivity in the excited state, which enabled us, after removal of the scaffold, to obtain a single enantiomer of chiral anti-head-to-head-cyclodimer in >99% optical and 96% chemical yield from an ensemble of four precursor conformers.
@article{fukuhara2015excited, title = {Excited-state dynamics achieved ultimate stereocontrol of photocyclodimerization of anthracenecarboxylates on a glucose scaffold}, author = {Fukuhara, Gaku and Iida, Kazuhiro and Kawanami, Yuko and Tanaka, Hidekazu and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Am. Chem. Soc.}, volume = {137}, number = {47}, pages = {15007--15014}, year = {2015}, publisher = {ACS Publications}, doi = {10.1021/jacs.5b09775}, url = {https://doi.org/10.1021/jacs.5b09775}, dimensions = {true}, tab = {paper}, } -
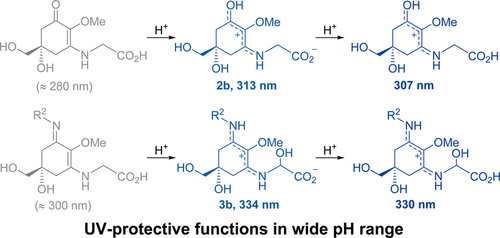 pH-independent charge resonance mechanism for UV protective functions of shinorine and related mycosporine-like amino acidsKeisuke Matsuyama, Jun Matsumoto, Shogo Yamamoto, Keisuke Nagasaki, Yoshihisa Inoue, Masaki Nishijima, and Tadashi Mori*J. Phys. Chem. A, 2015, 119, 12722–12729.
pH-independent charge resonance mechanism for UV protective functions of shinorine and related mycosporine-like amino acidsKeisuke Matsuyama, Jun Matsumoto, Shogo Yamamoto, Keisuke Nagasaki, Yoshihisa Inoue, Masaki Nishijima, and Tadashi Mori*J. Phys. Chem. A, 2015, 119, 12722–12729.The UV-protective ability of mycosporine-like amino acids (MAAs) has been well documented and is believed to serve as a protecting agent for marine organisms from solar radiation. However, the effective UV absorption by MAAs has not been well correlated to MAA (neutral) structures. In this study, the origin of UV-protecting ability of MAAs was elucidated by experimental and theoretical spectroscopic investigations. The absorption maxima of mycosporine–glycine and shinorine in the UVA region were practically unaffected over a wide range of pH 4–10 and only slightly blue-shifted at pH 1–2. It was revealed that the zwitterionic nature of the amino acid residue facilitates the protonation to the chromophoric 3-aminocyclohexenone and 1-amino-3-iminocyclohexene moieties and the operation of the charge resonance in the protonated species well accounts for their allowed low-energy transitions in the UVA region. The RI-CC2/TZVP calculations on model systems in their protonated forms well reproduced the observed transition energies and oscillator strengths of MAAs, only with insignificant systematic overestimations of the both values. The slight hypsochromic shifts at pH 1–2 were explained by (partial) protonation to a carboxylate anion in the amino acid residue, as confirmed by theory. Fluorescence spectral investigations of shinorine were also performed for the first time in water to confirm the effective nonradiative deactivation. Consequently, this study unequivocally demonstrated that the 3-aminocyclohexenone as well as 1-amino-3-iminocyclohexene moieties, which are readily protonated at a wide range of pH, are responsible for the UV-protective ability of aqueous solution of MAAs.
@article{matsuyama2015ph, title = {pH-independent charge resonance mechanism for UV protective functions of shinorine and related mycosporine-like amino acids}, author = {Matsuyama, Keisuke and Matsumoto, Jun and Yamamoto, Shogo and Nagasaki, Keisuke and Inoue, Yoshihisa and Nishijima, Masaki and Mori, Tadashi}, journal = {J. Phys. Chem. A}, volume = {119}, number = {51}, pages = {12722--12729}, year = {2015}, publisher = {ACS Publications}, doi = {10.1021/acs.jpca.5b09988}, url = {https://doi.org/10.1021/acs.jpca.5b09988}, dimensions = {true}, tab = {paper}, } -
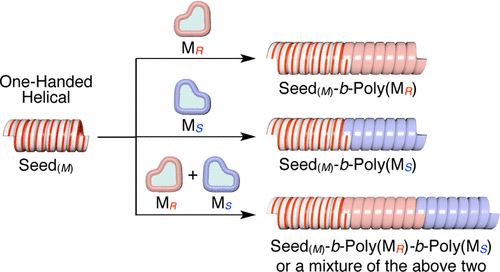 Helix sense-selective supramolecular polymerization seeded by a one-handed helical polymeric assemblyWei Zhang, Wusong Jin, Takanori Fukushima*, Tadashi Mori, and Takuzo Aida*. Am. Chem. Soc., 2015, 137, 13792–13795.
Helix sense-selective supramolecular polymerization seeded by a one-handed helical polymeric assemblyWei Zhang, Wusong Jin, Takanori Fukushima*, Tadashi Mori, and Takuzo Aida*. Am. Chem. Soc., 2015, 137, 13792–13795.Helix sense-selective supramolecular polymerization was achieved using a one-handed helical nanotubular polymeric assembly as a seed. First, bipyridine (BPY)-appended achiral hexabenzocoronene (BPYHBC) was copolymerized noncovalently with chiral BPYHBCS (or BPYHBCR) at a molar ratio of 9:1, which, via the sergeants-and-soldiers effect, afforded a P-helical (or M-helical) nanotube, which was then treated with Cu2+ to transform into structurally robust (BPY)CuNT(P) (or (BPY)CuNT(M)) with a Cu2+/BPY coordination polymer shell. Helical seeds (BPY)CuNT(P) and (BPY)CuNT(M) brought about the controlled assembly of fluorinated chiral FHBCS and FHBCR as well as achiral FHBC to yield one-handed helical nanotubular supramolecular block copolymers, in which the helical senses of the newly formed block segments were solely determined by those of the helical seeds employed. Noteworthy, FHBCS and FHBCR alone without the helical seeds form ill-defined agglomerates. Attempted supramolecular polymerization of a racemic mixture of FHBCS and FHBCR from (BPY)CuNT(P) (or (BPY)CuNT(M)) resulted in its chiral separation, affording P-helical (or M-helical) diastereomeric block segments composed of FHBCS and FHBCR with different thermodynamic properties.
@article{zhang2015helix, title = {Helix sense-selective supramolecular polymerization seeded by a one-handed helical polymeric assembly}, author = {Zhang, Wei and Jin, Wusong and Fukushima, Takanori and Mori, Tadashi and Aida, Takuzo}, journal = {. Am. Chem. Soc.}, volume = {137}, number = {43}, pages = {13792--13795}, year = {2015}, publisher = {ACS Publications}, doi = {10.1021/jacs.5b09878}, url = {https://doi.org/10.1021/jacs.5b09878}, dimensions = {true}, tab = {paper}, } -
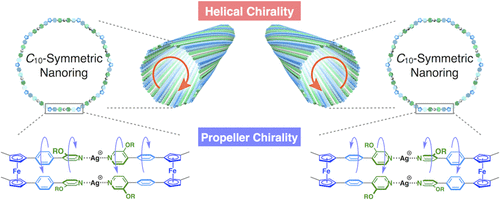 Metal–Organic Nanotube with Helical and Propeller-Chiral Motifs Composed of a C 10-Symmetric Double-Decker NanoringHiroshi Yamagishi, Takahiro Fukino*, Daisuke Hashizume, Tadashi Mori, Yoshihisa Inoue, Takaaki Hikima, Masaki Takata, and Takuzo Aida*J. Am. Chem. Soc., 2015, 137, 7628–7631.
Metal–Organic Nanotube with Helical and Propeller-Chiral Motifs Composed of a C 10-Symmetric Double-Decker NanoringHiroshi Yamagishi, Takahiro Fukino*, Daisuke Hashizume, Tadashi Mori, Yoshihisa Inoue, Takaaki Hikima, Masaki Takata, and Takuzo Aida*J. Am. Chem. Soc., 2015, 137, 7628–7631.Coassembly of an achiral ferrocene-cored tetratopic pyridyl ligand (FcL) with AgBF4 in CH2Cl2/MeCN (7:3 v/v) containing chiral Bu4N+ (+)- or (−)-menthylsulfate (MS*–) results in the formation of an “optically active” metal–organic nanotube (FcNT) composed of a C10-symmetric double-decker nanoring featuring 10 FcL units and 20 Ag+ ions. The circular dichroism spectrum of FcNT along with its 2D X-ray diffraction (2D XRD) pattern indicates that the constituent metal–organic nanorings in FcNT stack one-handed helically on top of each other. A crystal structure of the dimeric double-decker model complex (Ag2(FcL′)2) from a ditopic ferrocene ligand (FcL′) and AgBF4 allowed for confirming the binding of MS*– onto the Ag+ center of the complex. The results of detailed spectroscopic studies indicate that in its double-decker aromatic arrays, FcNT possibly possesses propeller-chiral twists in addition to the helically chiral structure, where the former is considerably more dynamic than the latter. Notably, both chiral structural motifs responded nonlinearly to an enantiomeric excess of MS*– (majority rule) though with no stereochemical influence on one another.
@article{yamagishi2015metal, title = {Metal--Organic Nanotube with Helical and Propeller-Chiral Motifs Composed of a C 10-Symmetric Double-Decker Nanoring}, author = {Yamagishi, Hiroshi and Fukino, Takahiro and Hashizume, Daisuke and Mori, Tadashi and Inoue, Yoshihisa and Hikima, Takaaki and Takata, Masaki and Aida, Takuzo}, journal = {J. Am. Chem. Soc.}, volume = {137}, number = {24}, pages = {7628--7631}, year = {2015}, publisher = {ACS Publications}, doi = {10.1021/jacs.5b04386}, url = {https://doi.org/10.1021/jacs.5b04386}, dimensions = {true}, tab = {paper}, } -
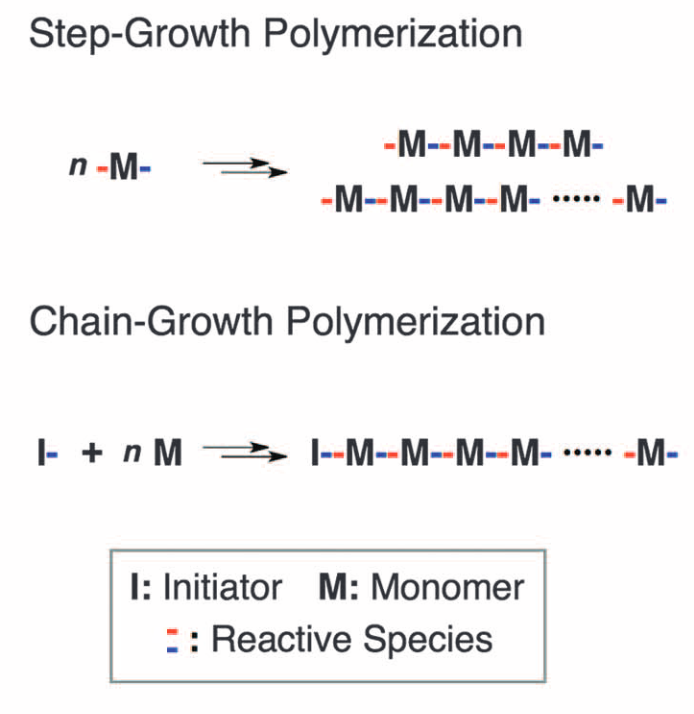 A rational strategy for the realization of chain-growth supramolecular polymerizationJiheong Kang, Daigo Miyajima*, Tadashi Mori, Yoshihisa Inoue, Yoshimitsu Itoh, and Takuzo Aida*Science, 2015, 347, 646–651.
A rational strategy for the realization of chain-growth supramolecular polymerizationJiheong Kang, Daigo Miyajima*, Tadashi Mori, Yoshihisa Inoue, Yoshimitsu Itoh, and Takuzo Aida*Science, 2015, 347, 646–651.Over the past decade, major progress in supramolecular polymerization has had a substantial effect on the design of functional soft materials. However, despite recent advances, most studies are still based on a preconceived notion that supramolecular polymerization follows a step-growth mechanism, which precludes control over chain length, sequence, and stereochemical structure. Here we report the realization of chain-growth polymerization by designing metastable monomers with a shape-promoted intramolecular hydrogen-bonding network. The monomers are conformationally restricted from spontaneous polymerization at ambient temperatures but begin to polymerize with characteristics typical of a living mechanism upon mixing with tailored initiators. The chain growth occurs stereoselectively and therefore enables optical resolution of a racemic monomer.
@article{kang2015rational, title = {A rational strategy for the realization of chain-growth supramolecular polymerization}, author = {Kang, Jiheong and Miyajima, Daigo and Mori, Tadashi and Inoue, Yoshihisa and Itoh, Yoshimitsu and Aida, Takuzo}, journal = {Science}, volume = {347}, number = {6222}, pages = {646--651}, year = {2015}, publisher = {American Association for the Advancement of Science}, doi = {10.1126/science.aaa4249}, url = {https://doi.org/10.1126/science.aaa4249}, dimensions = {true}, tab = {paper}, } -
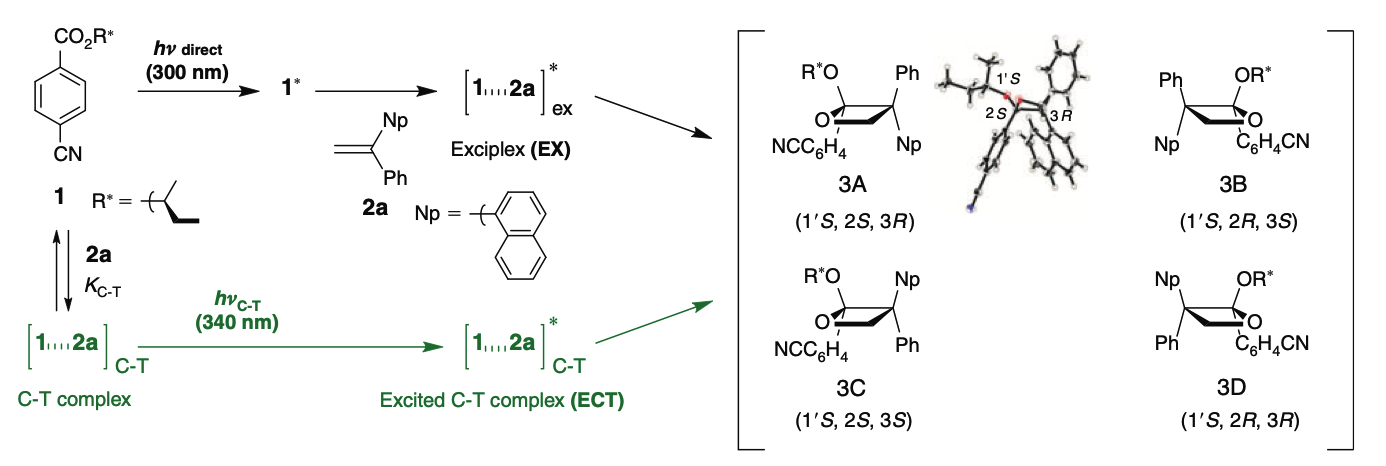 Contrasting Behaviour of Exciplex Ensembles in the Diastereodifferentiating Paternò–Büchi Reaction of Chiral Cyanobenzoate with Naphthyl-and Phenylethenes on Direct or Charge-Transfer ExcitationKeisuke Nagasaki, Yoshihisa Inoue, and Tadashi Mori*Aust. J. Chem., 2015, 68, 1693–1699.
Contrasting Behaviour of Exciplex Ensembles in the Diastereodifferentiating Paternò–Büchi Reaction of Chiral Cyanobenzoate with Naphthyl-and Phenylethenes on Direct or Charge-Transfer ExcitationKeisuke Nagasaki, Yoshihisa Inoue, and Tadashi Mori*Aust. J. Chem., 2015, 68, 1693–1699.The diastereodifferentiating Paternò–Büchi reaction of chiral cyanobenzoate with 1-(1-naphthyl)-1-phenylethene was compared with those with 1,1-diphenylethene on direct and charge-transfer excitations. By desymmetrization of the donor, four diastereomeric oxetane products were formed on photolysis in excellent combined yields. Increase in donor strength induced a stronger charge-transfer interaction both in ground and excited states. Thus, the difference in diastereoselectivities with two different excitation modes (i.e. direct versus charge-transfer) became less significant with a naphthyl derivative as donor. A subtle change of donor–acceptor interaction was shown to have profound effect on the nature of the excited-state complexes and thus the product (stereo)selectivities. Despite a small temperature dependence, an Eyring-type study on the diastereoselectivities confirmed that the excited charge-transfer complex is an excited species distinct from the conventional exciplex.
@article{nagasaki2015contrasting, title = {Contrasting Behaviour of Exciplex Ensembles in the Diastereodifferentiating Paternò--Büchi Reaction of Chiral Cyanobenzoate with Naphthyl-and Phenylethenes on Direct or Charge-Transfer Excitation}, author = {Nagasaki, Keisuke and Inoue, Yoshihisa and Mori, Tadashi}, journal = {Aust. J. Chem.}, volume = {68}, number = {11}, pages = {1693--1699}, year = {2015}, publisher = {CSIRO Publishing}, doi = {10.1071/ch15404}, url = {https://doi.org/10.1071/ch15404}, dimensions = {true}, tab = {paper}, } - Studies on Circular Dichroisms and Circular Polarized Luminescence Properties Based on Helical Structure of Helicene DerivativesTadashi Mori*Toyota Kenkyu Hokoku, 2015, 68, 173–174.
@article{mori2015studies, title = {Studies on Circular Dichroisms and Circular Polarized Luminescence Properties Based on Helical Structure of Helicene Derivatives}, author = {Mori, Tadashi}, journal = {Toyota Kenkyu Hokoku}, volume = {68}, pages = {173--174}, year = {2015} }