articles in 2013
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
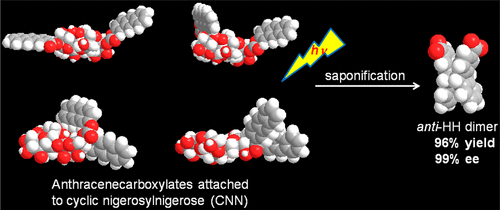 Diastereodifferentiating photocyclodimerization of 2-anthracenecarboxylates tethered to a cyclic tetrasaccharide scaffold: Critical control of photoreactivity and stereoselectivityGaku Fukuhara*, Tomohiro Nakamura, Yuko Kawanami, Cheng Yang, Tadashi Mori, Hiroyuki Hiramatsu, Yasufumi Dan-Oh, Tomoyuki Nishimoto, Kazuo Tsujimoto, and Yoshihisa Inoue*J. Org. Chem., 2013, 78, 10996–11006.
Diastereodifferentiating photocyclodimerization of 2-anthracenecarboxylates tethered to a cyclic tetrasaccharide scaffold: Critical control of photoreactivity and stereoselectivityGaku Fukuhara*, Tomohiro Nakamura, Yuko Kawanami, Cheng Yang, Tadashi Mori, Hiroyuki Hiramatsu, Yasufumi Dan-Oh, Tomoyuki Nishimoto, Kazuo Tsujimoto, and Yoshihisa Inoue*J. Org. Chem., 2013, 78, 10996–11006.From a complex mixture of mono- and di-2-anthracenecarboxylic acid (AC) esters of cyclic nigerosylnigerose (CNN), two monoesters (2B and 6A) and four diesters in which AC was introduced on the transannular B/D (2B2D), adjacent A/B and A/D (6A2B and 6A2D), and same B/B (2B3B) nigerose rings were isolated. Possessing two ACs at distant positions, 2B2D and 6A2D showed negative Cotton effects for the 1Bb band, the intensities of which were stronger than that of 6A. 2B2D and 6A2D slowly photocyclodimerized to give HH dimers 3* and 4 with 57% and 81% HH selectivity, respectively, which were appreciably higher than that for 6A (34%), while the enantiomeric excesses (ee’s) of anti-HH dimer 3* were 2% and −18%, respectively. In contrast, 6A2B and 2B3B carrying two ACs on adjacent A and B rings or at vicinal positions on the B ring, respectively, exhibited strong positive CD couplets, the amplitudes of which amounted to 97 and 409 M–1 cm–1, respectively. Upon irradiation, 6A2B afforded 3* with −62% ee and 4 in 96% combined yield, whereas 2B3B gave almost exclusively 3* with −99% ee in 96% yield, likely as a result of the introduction of two ACs at the vicinal positions of the rigid CNN scaffold.
@article{fukuhara2013diastereodifferentiating, title = {Diastereodifferentiating photocyclodimerization of 2-anthracenecarboxylates tethered to a cyclic tetrasaccharide scaffold: Critical control of photoreactivity and stereoselectivity}, author = {Fukuhara, Gaku and Nakamura, Tomohiro and Kawanami, Yuko and Yang, Cheng and Mori, Tadashi and Hiramatsu, Hiroyuki and Dan-Oh, Yasufumi and Nishimoto, Tomoyuki and Tsujimoto, Kazuo and Inoue, Yoshihisa}, journal = {J. Org. Chem.}, volume = {78}, number = {21}, pages = {10996--11006}, year = {2013}, publisher = {ACS Publications}, doi = {10.1021/jo401977f}, url = {https://doi.org/10.1021/jo401977f}, dimensions = {true}, tab = {paper}, } -
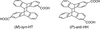 Absolute configuration determination of the anti-head-to-head photocyclodimer of anthracene-2-carboxylic acid through cocrystallization with l-prolinolYuko Kawanami, Hidekazu Tanaka, Jun-ichi Mizoguchi, Nobuko Kanehisa, Gaku Fukuhara, Masaki Nishijima, Tadashi Mori, and Yoshihisa InoueActa Cryst. C, 2013, 69, 1411–1413.
Absolute configuration determination of the anti-head-to-head photocyclodimer of anthracene-2-carboxylic acid through cocrystallization with l-prolinolYuko Kawanami, Hidekazu Tanaka, Jun-ichi Mizoguchi, Nobuko Kanehisa, Gaku Fukuhara, Masaki Nishijima, Tadashi Mori, and Yoshihisa InoueActa Cryst. C, 2013, 69, 1411–1413.The absolute configuration has been established of the enantio pure anti-head-to-head cyclo dimer of anthracene-2-carboxylic acid (AC) cocrystallized with L-propinol and dichloromethane [systematic name: (S)-2-(hydroxymethyl)pyrrolidin-1-ium (5R,6S,11R,12S)-8-carboxy-5,6,11,12-tetrahydro-5,12:6,11-bis([1,2]benzeno)dibenzo[a,e][8]annulene-2-carboxylate dichloromethane monosolvate], C5H12NO+·C30H19O4-·CH2Cl2. In the crystal structure, the AC dimer interacts with L-prolinol through a nine-membered hydrogen-bonded ring [R22(9)], while the dichloromethane molecule is incorporated to fill the void space. The absolute configuration determined in this study verifies a recent assignment made by comparing theoretical versus experimental circular dichroism spectra.
@article{kawanami2013absolute, title = {Absolute configuration determination of the anti-head-to-head photocyclodimer of anthracene-2-carboxylic acid through cocrystallization with l-prolinol}, author = {Kawanami, Yuko and Tanaka, Hidekazu and Mizoguchi, Jun-ichi and Kanehisa, Nobuko and Fukuhara, Gaku and Nishijima, Masaki and Mori, Tadashi and Inoue, Yoshihisa}, journal = {Acta Cryst. C}, volume = {69}, number = {11}, pages = {1411--1413}, year = {2013}, publisher = {International Union of Crystallography}, doi = {10.1107/S0108270113028461}, url = {https://doi.org/10.1107/S0108270113028461}, dimensions = {true}, tab = {paper}, } -
 Catalytic Bio-Supramolecular Photochirogenesis: Batch-Operated Enantiodifferentiating Photocyclodimerization of 2-Anthracenecarboxylate with Human Serum Albumin.Masaki Nishijima, Hanako Kato, Cheng Yang, Gaku Fukuhara, Tadashi Mori, Yasuyuki Araki, Takehiko Wada, and Yoshihisa Inoue*ChemCatChem, 2013, 5, 1411–1413.
Catalytic Bio-Supramolecular Photochirogenesis: Batch-Operated Enantiodifferentiating Photocyclodimerization of 2-Anthracenecarboxylate with Human Serum Albumin.Masaki Nishijima, Hanako Kato, Cheng Yang, Gaku Fukuhara, Tadashi Mori, Yasuyuki Araki, Takehiko Wada, and Yoshihisa Inoue*ChemCatChem, 2013, 5, 1411–1413.Catalytic bio-supramolecular photochirogenesis is achieved for the enantiodifferentiating [4+4] photocyclodimerization of 2-anthracenecarboxylate by repeatedly using human serum albumin (HSA) as a chiral mediator; the original high enantiomeric excess values (up to 80–90%) are preserved.
@article{nishijima2013catalytic, title = {Catalytic Bio-Supramolecular Photochirogenesis: Batch-Operated Enantiodifferentiating Photocyclodimerization of 2-Anthracenecarboxylate with Human Serum Albumin.}, author = {Nishijima, Masaki and Kato, Hanako and Yang, Cheng and Fukuhara, Gaku and Mori, Tadashi and Araki, Yasuyuki and Wada, Takehiko and Inoue, Yoshihisa}, journal = {ChemCatChem}, volume = {5}, number = {11}, year = {2013}, doi = {10.1002/cctc.201300160}, url = {https://doi.org/10.1002/cctc.201300160}, dimensions = {true}, tab = {paper}, } -
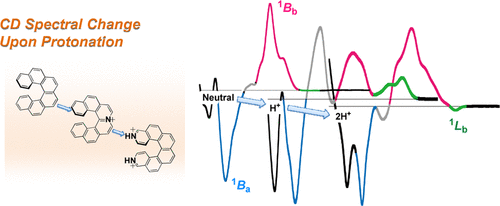 Theoretical and experimental studies of circular dichroism of mono-and diazonia [6] helicenesYoshito Nakai, Tadashi Mori*, Kiyoshi Sato, and Yoshihisa Inoue*J. Phys. Chem. A, 2013, 117, 5082–5092.
Theoretical and experimental studies of circular dichroism of mono-and diazonia [6] helicenesYoshito Nakai, Tadashi Mori*, Kiyoshi Sato, and Yoshihisa Inoue*J. Phys. Chem. A, 2013, 117, 5082–5092.Combined experimental and theoretical studies revealed the characteristic circular dichroism (CD) spectral profiles of mono- and diazonia[6]helicenes, which were distinctly different from those reported for parent [6]helicene and neutral (di)aza-analogues. Aza[6]helicenes and [6]helicene showed bisignate Cotton effects (CEs) at the 1Ba and 1Bb bands, along with a weak CE at the 1Lb band, where the signs of the former bands are responsible for the helical chirality of the helicenes while the sign of the latter is susceptive to the various factors such as electronic and steric effects. Protonation to monoaza[6]helicenes produces azonia[6]helicenes, showing dramatic changes in the CE pattern from the two bisignate to a three positive, two negative CE extremum series of comparable magnitudes, while dual protonation to diaza[6]helicenes forming diazonia[6]helicenes led to only nominal changes (slightly different rotational strength and excitation energy) in the CE pattern. Such rather complicated and contrasting CE behaviors of mono- versus diazoniahelicenes are derived mostly from the electronic effects of (unsymmetrical) protonation because the structures of neutral, mono-, and dicationic species are essentially identical to each other. Compared with those of neutral (di)aza[6]helicenes, the experimental CD spectra of (di)azonia[6]helicenes were less satisfactorily reproduced by the theoretical calculations at the state-of-the-art RI-CC2/TZVPP//DFT-D2-B97-D/TZVP level, most probably due to the inadequate incorporation of the effects of solvation. Nevertheless, the bytheoretical predictions were reasonably accurate and highly valuable in assigning the observed CE and elucidating the origin of the elaborate CD spectral behaviors upon protonation through inspection of the molecular orbital configuration of each transition, encouraging the extended use of the present protocol for analyzing the CD spectral behavior of aza- and other heteroatom-incorporated helicenes upon protonation. The CD spectral behavior upon metal ligation will also be explained through further theoretical and experimental studies.
@article{nakai2013theoretical, title = {Theoretical and experimental studies of circular dichroism of mono-and diazonia [6] helicenes}, author = {Nakai, Yoshito and Mori, Tadashi and Sato, Kiyoshi and Inoue, Yoshihisa}, journal = {J. Phys. Chem. A}, volume = {117}, number = {24}, pages = {5082--5092}, year = {2013}, publisher = {ACS Publications}, doi = {10.1021/jp403426w}, url = {https://doi.org/10.1021/jp403426w}, dimensions = {true}, tab = {paper}, } -
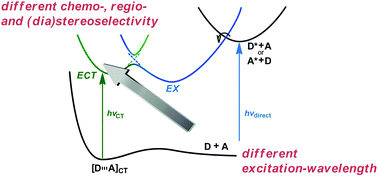 Charge-transfer excitation: unconventional yet practical means for controlling stereoselectivity in asymmetric photoreactionsTadashi Mori* and Yoshihisa InoueChem. Soc. Rev., 2013, 42, 8122–8133.
Charge-transfer excitation: unconventional yet practical means for controlling stereoselectivity in asymmetric photoreactionsTadashi Mori* and Yoshihisa InoueChem. Soc. Rev., 2013, 42, 8122–8133.In chiral donor–acceptor (D–A) systems, irradiation wavelength plays vital roles in determining the photochemical consequences. Selective excitation of a D–A complex at the charge-transfer (C-T) band affords an excited C-T complex (ECT), while the local-band excitation of D or A may lead to the formation of a conventional exciplex (EX) upon subsequent interaction with the D–A partner. These two excited species, generated from the same D–A pair, may be categorized formally as excited complexes or exciplexes, but should be distinguished, provided that they significantly differ in structure and reactivity. Indeed, ECT and EX exhibit distinctly different temperature-dependent photophysical and photochemical behaviours, which are assignable to the differences in relative stability, conformational flexibility and/or solvation properties. Fine-tuning excitation wavelength further enabled us to discriminate stereoisomeric intramolecular C-T complexes through preferential excitation, as C-T complexes are generally composed of an ensemble of various geometries. Besides temperature and solvent polarity, the excitation wavelength was shown to be employed as an unconventional yet practical tool for critically controlling the chemo-, regio- and stereoselectivities in molecular and supramolecular photochemistry.
@article{mori2013charge, title = {Charge-transfer excitation: unconventional yet practical means for controlling stereoselectivity in asymmetric photoreactions}, author = {Mori, Tadashi and Inoue, Yoshihisa}, journal = {Chem. Soc. Rev.}, volume = {42}, number = {20}, pages = {8122--8133}, year = {2013}, publisher = {Royal Society of Chemistry}, doi = {10.1039/C3CS60117J}, url = {https://doi.org/10.1039/C3CS60117J}, dimensions = {true}, tab = {review}, } -
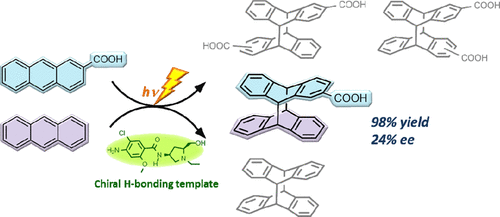 Cross-versus homo-photocyclodimerization of anthracene and 2-anthracenecarboxylic acid mediated by a chiral hydrogen-bonding template. Factors controlling the cross-/homo-selectivity and enantioselectivityYuko Kawanami, Hiroaki Umehara, Jun-ichi Mizoguchi, Masaki Nishijima, Gaku Fukuhara, Cheng Yang, Tadashi Mori, and Yoshihisa Inoue*J. Org. Chem., 2013, 78, 3073–3085.
Cross-versus homo-photocyclodimerization of anthracene and 2-anthracenecarboxylic acid mediated by a chiral hydrogen-bonding template. Factors controlling the cross-/homo-selectivity and enantioselectivityYuko Kawanami, Hiroaki Umehara, Jun-ichi Mizoguchi, Masaki Nishijima, Gaku Fukuhara, Cheng Yang, Tadashi Mori, and Yoshihisa Inoue*J. Org. Chem., 2013, 78, 3073–3085.Competitive cross-/homo-photocyclodimerization of anthracene (AN) and 2-anthracenecarboxylic acid (AC) mediated by a chiral hydrogen-bonding template (TKS) was investigated under various conditions. The cross-photocyclodimerization was favored by a factor of 4–5 at all temperatures and wavelengths examined to afford the AC-AN cross-dimer in 80–84% yield even at AN/AC = 1 and in 98% yield at AN/AC = 10. The enantiomeric excesses (ee’s) obtained were 27–47% for the homo-dimers and 21–24% for the cross-dimer. The absolute configuration of the cross-dimer was determined by comparing the experimental and theoretical circular dichroism spectra and further correlated with the re/si enantiotopic-face selectivity upon AC-TKS complexation in the ground state. Detailed analyses of the complexation behavior and the fluorescence lifetime and cyclodimerization rate of excited re/si complexes revealed that the product’s ee is critically controlled not only by the relative abundance of the re/si complexes in the ground and excited states but also by their relative photocyclodimerization rate. Crucially, the ground-state thermodynamics and the excited-state kinetics are not synergistic but offsetting in enantiotopic-face selectivity, and the latter overwhelms the former to give the homo- and cross-dimers in modest ee’s. Finally, some practical strategies for enhancing the enantioselectivity in chiral template-mediated photochirogenesis have been proposed.
@article{kawanami2013cross, title = {Cross-versus homo-photocyclodimerization of anthracene and 2-anthracenecarboxylic acid mediated by a chiral hydrogen-bonding template. Factors controlling the cross-/homo-selectivity and enantioselectivity}, author = {Kawanami, Yuko and Umehara, Hiroaki and Mizoguchi, Jun-ichi and Nishijima, Masaki and Fukuhara, Gaku and Yang, Cheng and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Org. Chem.}, volume = {78}, number = {7}, pages = {3073--3085}, year = {2013}, publisher = {ACS Publications}, doi = {10.1021/jo302818w}, url = {https://doi.org/10.1021/jo302818w}, dimensions = {true}, tab = {paper}, } -
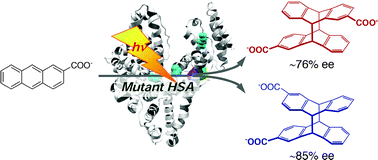 Photochirogenesis with mutant human serum albumins: enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylateMasaki Nishijima, Hanako Kato, Gaku Fukuhara, Cheng Yang, Tadashi Mori, Toru Maruyama, Masaki Otagiri*, and Yoshihisa Inoue*Chem. Commun., 2013, 49, 7433–7435.
Photochirogenesis with mutant human serum albumins: enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylateMasaki Nishijima, Hanako Kato, Gaku Fukuhara, Cheng Yang, Tadashi Mori, Toru Maruyama, Masaki Otagiri*, and Yoshihisa Inoue*Chem. Commun., 2013, 49, 7433–7435.Mutant human serum albumins accelerated the photocyclodimerization of 2-anthracenecarboxylate to afford chiral cyclodimers in 75–85% enantiomeric excesses, revealing that the mutations to impair non-productive sites 1 and/or 2 enhanced the substrate binding to site 3 without seriously damaging its inherently high photochirogenic ability.
@article{nishijima2013photochirogenesis, title = {Photochirogenesis with mutant human serum albumins: enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate}, author = {Nishijima, Masaki and Kato, Hanako and Fukuhara, Gaku and Yang, Cheng and Mori, Tadashi and Maruyama, Toru and Otagiri, Masaki and Inoue, Yoshihisa}, journal = {Chem. Commun.}, volume = {49}, number = {67}, pages = {7433--7435}, year = {2013}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c3cc42656d}, url = {https://doi.org/10.1039/c3cc42656d}, dimensions = {true}, tab = {paper}, } -
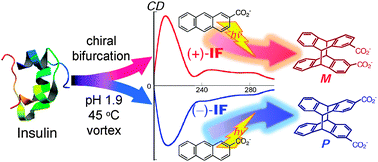 Supramolecular photochirogenesis with functional amyloid superstructuresMasaki Nishijima, Hidekazu Tanaka, Cheng Yang, Gaku Fukuhara, Tadashi Mori, Viktoria Babenko, Wojciech Dzwolak*, and Yoshihisa Inoue*Chem. Commun., 2013, 49, 8916–8918.
Supramolecular photochirogenesis with functional amyloid superstructuresMasaki Nishijima, Hidekazu Tanaka, Cheng Yang, Gaku Fukuhara, Tadashi Mori, Viktoria Babenko, Wojciech Dzwolak*, and Yoshihisa Inoue*Chem. Commun., 2013, 49, 8916–8918.Chiral variants of amyloid fibrils prepared by agitating acidified solutions of bovine insulin at 45 °C not only induced quasi-mirror-imaged circular dichroism spectra upon complexation with 2-anthracenecarboxylate but also gave anti-head-to-head-cyclodimers of the opposite absolute configurations upon photoirradiation.
@article{nishijima2013supramolecular, title = {Supramolecular photochirogenesis with functional amyloid superstructures}, author = {Nishijima, Masaki and Tanaka, Hidekazu and Yang, Cheng and Fukuhara, Gaku and Mori, Tadashi and Babenko, Viktoria and Dzwolak, Wojciech and Inoue, Yoshihisa}, journal = {Chem. Commun.}, volume = {49}, number = {79}, pages = {8916--8918}, year = {2013}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c3cc44235g}, url = {https://doi.org/10.1039/c3cc44235g}, dimensions = {true}, tab = {paper}, } -
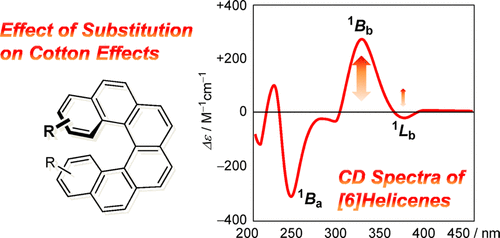 Circular dichroism of (di) methyl-and diaza [6] helicenes. A combined theoretical and experimental studyYoshito Nakai, Tadashi Mori*, and Yoshihisa Inoue*J. Phys. Chem. A, 2013, 117, 83–93.
Circular dichroism of (di) methyl-and diaza [6] helicenes. A combined theoretical and experimental studyYoshito Nakai, Tadashi Mori*, and Yoshihisa Inoue*J. Phys. Chem. A, 2013, 117, 83–93.Circular dichroism (CD) and relevant chiroptical properties of (di)methyl- and diaza[6]helicenes were investigated by the state-of-the-art approximate coupled cluster and density functional theory calculations, results of which were compared with the corresponding experimental data obtained for newly synthesized enantiopure helicenes. The theoretical calculation at the RI-CC2/TZVPP//DFT-D2-B97-D/TZVP level accurately reproduced the experimental CD spectra in both excitation energy and rotational strength. The electric and magnetic transition dipole moment vectors for the helical sense-responsive 1Bb and the substitution-sensitive 1Lb bands were compared with those for parent carbo[6]helicene, from which the effects of methyl and nitrogen introduced at different positions upon the experimental CD spectra were discussed to separately evaluate the electronic and steric consequences of the substitution to the chiroptical properties. The electronic effects of substitution on CD spectra were further investigated theoretically by employing a series of 3,3-disubstituted [6]helicenes. This first systematic investigation allows us not only to accurately reproduce the experimental CD spectra of known substituted helicenes but also to directly envisage the chiroptical properties of unknown helicenes.
@article{nakai2013circular, title = {Circular dichroism of (di) methyl-and diaza [6] helicenes. A combined theoretical and experimental study}, author = {Nakai, Yoshito and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Phys. Chem. A}, volume = {117}, number = {1}, pages = {83--93}, year = {2013}, publisher = {ACS Publications}, doi = {10.1021/jp3104084}, url = {https://doi.org/10.1021/jp3104084}, dimensions = {true}, tab = {paper}, } -
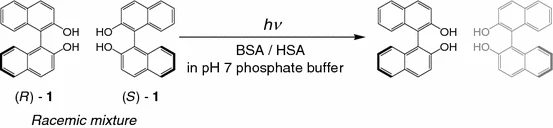 Chiral recognition and supramolecular photoreaction of 1, 1′-binaphthol with bovine and human serum albuminsMasaki Nishijima, Jae-Won Chang, Cheng Yang, Gaku Fukuhara, Tadashi Mori, and Yoshihisa Inoue*Res. Chem. Intermed., 2013, 39, 371–383.
Chiral recognition and supramolecular photoreaction of 1, 1′-binaphthol with bovine and human serum albuminsMasaki Nishijima, Jae-Won Chang, Cheng Yang, Gaku Fukuhara, Tadashi Mori, and Yoshihisa Inoue*Res. Chem. Intermed., 2013, 39, 371–383.In their pioneering study in 1991, Levi-Minzi and Zandomeneghi discovered that photoirradiation of racemic 1,1′-binaphthol (rac-1) in the presence of bovine serum albumin (BSA) in distilled water gave (R)-1 in 99 % enantiomeric excess (ee) after 77 % of the starting material had been consumed. No similar attempt was made with human serum albumin (HSA). In this study of the effects of phosphate buffer solution on the ground-state affinity and excited-state photobehavior of 1 with serum albumin we found that both BSA and HSA preferentially bind the (S) enantiomer of 1 and that photoreaction of rac-1 mediated by BSA and HSA affords (R)-1 in 98 % ee with 99 % conversion and in 46 % ee with 65 % conversion, respectively.
@article{nishijima2013chiral, title = {Chiral recognition and supramolecular photoreaction of 1, 1′-binaphthol with bovine and human serum albumins}, author = {Nishijima, Masaki and Chang, Jae-Won and Yang, Cheng and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa}, journal = {Res. Chem. Intermed.}, volume = {39}, number = {1}, pages = {371--383}, year = {2013}, publisher = {Springer}, doi = {10.1007/s11164-012-0655-1}, url = {https://doi.org/10.1007/s11164-012-0655-1}, dimensions = {true}, tab = {paper}, } -
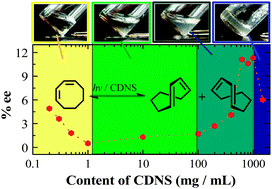 Phase-controlled supramolecular photochirogenesis in cyclodextrin nanospongesWenting Liang, Cheng Yang*, Dayang Zhou, Hitoshi Haneoka, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Franca Castiglione, Andrea Mele*, Fabrizio Caldera, Francesco Trotta*, and Yoshihisa Inoeu*Chem. Commun., 2013, 49, 3510–3512.
Phase-controlled supramolecular photochirogenesis in cyclodextrin nanospongesWenting Liang, Cheng Yang*, Dayang Zhou, Hitoshi Haneoka, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Franca Castiglione, Andrea Mele*, Fabrizio Caldera, Francesco Trotta*, and Yoshihisa Inoeu*Chem. Commun., 2013, 49, 3510–3512.Pyromellitate-bridged cyclodextrin nanosponges (CDNSs) evolved from sol into gel state upon gradual increase of the concentration from 0.2 to 2000 mg mL−1 in water. The enantiodifferentiating geometrical photoisomerizations of (Z)-cyclooctene and (Z,Z)-1,3-cyclooctadiene sensitized by CDNS at various concentrations were critically affected by the phase transition of CDNS to afford the corresponding (E)- and (E,Z)-isomers in the highest enantiomeric excesses in the gel state.
@article{liang2013phase, title = {Phase-controlled supramolecular photochirogenesis in cyclodextrin nanosponges}, author = {Liang, Wenting and Yang, Cheng and Zhou, Dayang and Haneoka, Hitoshi and Nishijima, Masaki and Fukuhara, Gaku and Mori, Tadashi and Castiglione, Franca and Mele, Andrea and Caldera, Fabrizio and Trotta, Francesco and Inoeu, Yoshihisa}, journal = {Chem. Commun.}, volume = {49}, number = {34}, pages = {3510--3512}, year = {2013}, publisher = {Royal Society of Chemistry}, doi = {10.1039/C3CC40542G}, url = {https://doi.org/10.1039/C3CC40542G}, dimensions = {true}, tab = {paper}, } -
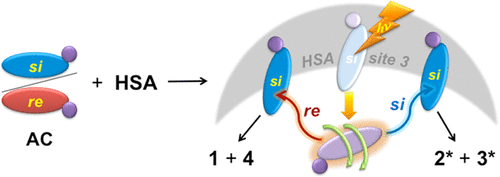 Explaining the highly enantiomeric photocyclodimerization of 2-anthracenecarboxylate bound to human serum albumin using time-resolved anisotropy studiesDenis Fuentealba, Hanako Kato, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cornelia Bohne*J. Am. Chem. Soc., 2013, 135, 203–209.
Explaining the highly enantiomeric photocyclodimerization of 2-anthracenecarboxylate bound to human serum albumin using time-resolved anisotropy studiesDenis Fuentealba, Hanako Kato, Masaki Nishijima, Gaku Fukuhara, Tadashi Mori, Yoshihisa Inoue*, and Cornelia Bohne*J. Am. Chem. Soc., 2013, 135, 203–209.The mechanism for the high enantiomeric excess (ee) (80–90%) observed in the photocyclodimerization of 2-anthracenecarboxylate (AC) in the chiral binding sites of human serum albumin (HSA) was studied using fluorescence anisotropy. A long rotational correlation time of 36 ns was observed for the excited states of the ACs bound to the HSA site responsible for the high ee, suggesting that the ACs have restricted rotational mobility in this site. The ACs in this site have the same prochiral face protected by the protein, and this protection is responsible for the high ee observed. These insights provide a strategy for the rational design of supramolecular photochirogenic systems.
@article{fuentealba2013explaining, title = {Explaining the highly enantiomeric photocyclodimerization of 2-anthracenecarboxylate bound to human serum albumin using time-resolved anisotropy studies}, author = {Fuentealba, Denis and Kato, Hanako and Nishijima, Masaki and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa and Bohne, Cornelia}, journal = {J. Am. Chem. Soc.}, volume = {135}, number = {1}, pages = {203--209}, year = {2013}, publisher = {ACS Publications}, doi = {10.1021/ja3081555}, url = {https://doi.org/10.1021/ja3081555}, dimensions = {true}, tab = {paper}, }