articles in 2011
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
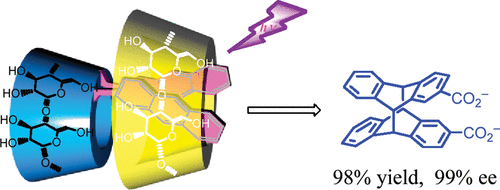 Dual supramolecular photochirogenesis: ultimate stereocontrol of photocyclodimerization by a chiral scaffold and confining hostCheng Yang, Chenfeng Ke, Wenting Liang, Gaku Fukuhara, Tadashi Mori, Yu Liu, and Yoshihisa Inoue*J. Am. Chem. Soc., 2011, 133, 13786–13789.
Dual supramolecular photochirogenesis: ultimate stereocontrol of photocyclodimerization by a chiral scaffold and confining hostCheng Yang, Chenfeng Ke, Wenting Liang, Gaku Fukuhara, Tadashi Mori, Yu Liu, and Yoshihisa Inoue*J. Am. Chem. Soc., 2011, 133, 13786–13789.In contrast to the brilliant success in thermal asymmetric synthesis, precise stereocontrol remains a great challenge in chiral photochemistry because of the lack of effective tools and methodologies for controlling the short-lived, weakly interacting, and highly reactive electronically excited species. In this work, we achieved this goal through the “dual-chiral, dual-supramolecular” photochirogenesis approach, which enabled us to realized dramatic acceleration and perfect stereocontrol in one of the most representative photoreactions. Thus, the [4 + 4] photocyclodimerization of 2-anthracenecarboxylate tethered to an α-cyclodextrin scaffold was accelerated by a γ-cyclodextrin or cucurbit[8]uril host and gave a single enantiomeric cyclodimer (out of four possible chiral and achiral stereoisomers) in up to 98% chemical and 99% optical yield.
@article{yang2011dual, title = {Dual supramolecular photochirogenesis: ultimate stereocontrol of photocyclodimerization by a chiral scaffold and confining host}, author = {Yang, Cheng and Ke, Chenfeng and Liang, Wenting and Fukuhara, Gaku and Mori, Tadashi and Liu, Yu and Inoue, Yoshihisa}, journal = {J. Am. Chem. Soc.}, volume = {133}, number = {35}, pages = {13786--13789}, year = {2011}, publisher = {ACS Publications}, doi = {10.1021/ja202020x}, url = {https://doi.org/10.1021/ja202020x}, dimensions = {true}, tab = {paper}, } -
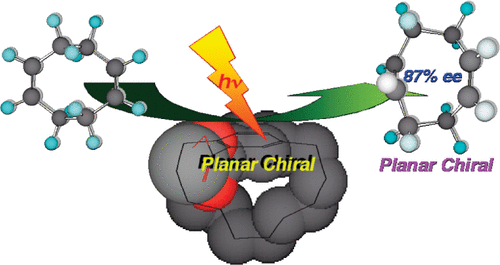 Planar-to-planar chirality transfer in the excited state. Enantiodifferentiating photoisomerization of cyclooctenes sensitized by planar-chiral paracyclophaneRyo Maeda, Takehiko Wada, Tadashi Mori, Shigeyuki Kono, Nobuhiro Kanomata*, and Yoshihisa Inoue*J. Am. Chem. Soc., 2011, 133, 10379–10381.
Planar-to-planar chirality transfer in the excited state. Enantiodifferentiating photoisomerization of cyclooctenes sensitized by planar-chiral paracyclophaneRyo Maeda, Takehiko Wada, Tadashi Mori, Shigeyuki Kono, Nobuhiro Kanomata*, and Yoshihisa Inoue*J. Am. Chem. Soc., 2011, 133, 10379–10381.Photochemical planar-to-planar chirality transfer was effected by using (R)-[10]paracyclophane-12-carboxylates as a planar-chiral sensitizer and (Z)-cyclooctene and (Z,Z)-1,5-cyclooctadiene as prochiral substrates to give a planar-chiral (E)- and (E,Z)-isomer in up to 44% and 87% enantiomeric excess, respectively, the latter of which being the highest ever reported for a sensitized photochirogenic reaction.
@article{maeda2011planar, title = {Planar-to-planar chirality transfer in the excited state. Enantiodifferentiating photoisomerization of cyclooctenes sensitized by planar-chiral paracyclophane}, author = {Maeda, Ryo and Wada, Takehiko and Mori, Tadashi and Kono, Shigeyuki and Kanomata, Nobuhiro and Inoue, Yoshihisa}, journal = {J. Am. Chem. Soc.}, volume = {133}, number = {27}, pages = {10379--10381}, year = {2011}, publisher = {ACS Publications}, doi = {10.1021/ja203781f}, url = {https://doi.org/10.1021/ja203781f}, dimensions = {true}, tab = {paper}, } -
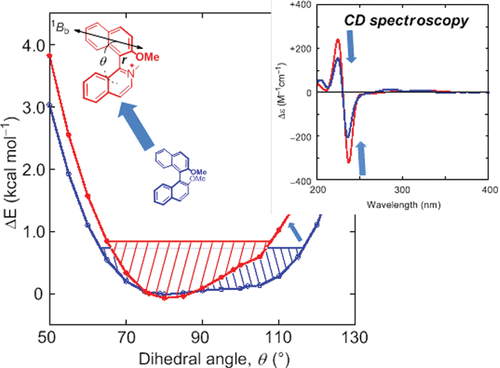 Axial chirality of donor–donor, donor–acceptor, and tethered 1, 1′-binaphthyls: a theoretical revisit with dynamics trajectoriesMasaki Nishizaka, Tadashi Mori*, and Yoshihisa Inoue*J. Phys. Chem. A, 2011, 115, 5488–5495.
Axial chirality of donor–donor, donor–acceptor, and tethered 1, 1′-binaphthyls: a theoretical revisit with dynamics trajectoriesMasaki Nishizaka, Tadashi Mori*, and Yoshihisa Inoue*J. Phys. Chem. A, 2011, 115, 5488–5495.The circular dichroism (CD) spectra of (R)-2,2′-dimethoxy-1,1′-binaphthyl (DD) and its untethered and tethered donor–acceptor analogues (DA and DA7–DA9) were investigated experimentally and theoretically. The experimental CD spectra of DD and DA resembled each other in several aspects, displaying a positive–positive–negative Cotton effect pattern in the 1Lb–1La region and a strong negative couplet at the 1Bb band, but significantly differed in transition energy and rotatory strength. The couplet amplitude (A) of the main band was 1.6 times larger in DA than in DD, despite the comparable extinction coefficients and seemingly analogous conformations. An additional positive Cotton effect was observed at the CT (CT) band for donor–acceptor binaphthyl DA. Our theoretical prediction of the CD spectra of binaphthyls involves three sequential first principle quantum mechanics (QM) calculations. Thus, the geometry optimizations of a series of conformers with varying dihedral angles were performed by the dispersion-corrected DFT-D method using the B97-D functional and the TZV2P basis set. The potential curve as a function of the dihedral angle (θ) was obtained by using the SCS-MP2/TZVPP single-point energy calculations with and without application of the solvent correction. The CD spectrum of each conformer was independently calculated by the second-order approximate coupled cluster calculation (CC2 method) using the TZVPP basis sets and the resolution of the identity (RI-J) approximation. The (net) theoretical CD spectrum was obtained by averaging over all possible conformers, where the dynamics trajectories based on the relative SCS-MP2 energies were taken into account. By using 17 possible conformers at θ varying from 50 to 130° by 5° intervals, the experimental CD spectra were successfully reproduced in a quantitative manner, enabling us to characterize properly almost all of the important spectral features and chiroptical properties. The two-state model, reported previously, turned out to have led to the right answer with wrong reasons. The couplet sign and amplitude A are critical functions of θ and can be used not only for (qualitatively) determining the absolute configuration but also for quantitatively analyzing the binaphthyl conformations. The angle dependence of A was already argued in the classical coupled oscillator and exciton chirality theories to provide reasonable structure elucidations but only in a qualitative or semiquantitative manner. Our method is able to predict the A value quantitatively as a function of θ. For tethered binaphthyls DA7–DA9, particular care should be exercised in the conformational assessment based on the classical treatment because the amplitude A was shown to be significantly affected by the existence of the tether itself. In the present method, the couplet amplitude A was nicely related to the dihedral angle θ of DA and DD by the state-of-the-art ab initio calculations, enabling us to gain the quantitative information about the conformation of axially chiral binaphthyls. The Cotton effect at the CT band also serves as a complementary clue for elucidating the conformation of donor–acceptor binaphthyls.
@article{nishizaka2011axial, title = {Axial chirality of donor--donor, donor--acceptor, and tethered 1, 1′-binaphthyls: a theoretical revisit with dynamics trajectories}, author = {Nishizaka, Masaki and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Phys. Chem. A}, volume = {115}, number = {21}, pages = {5488--5495}, year = {2011}, publisher = {ACS Publications}, doi = {10.1021/jp202776g}, url = {https://doi.org/10.1021/jp202776g}, dimensions = {true}, tab = {paper}, } -
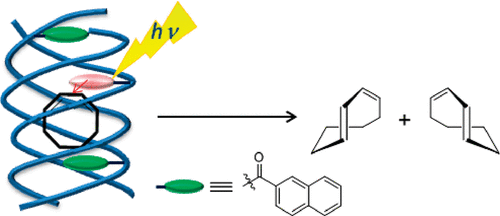 Enantiodifferentiating photoisomerization of (Z, Z)-1, 3-cyclooctadiene included and sensitized by naphthoyl-curdlanGaku Fukuhara*, Mami Imai, Cheng Yang, Tadashi Mori, and Yoshihisa Inoue*Org. Lett., 2011, 13, 1856–1859.
Enantiodifferentiating photoisomerization of (Z, Z)-1, 3-cyclooctadiene included and sensitized by naphthoyl-curdlanGaku Fukuhara*, Mami Imai, Cheng Yang, Tadashi Mori, and Yoshihisa Inoue*Org. Lett., 2011, 13, 1856–1859.6-O-(2-Naphthoyl)curdlan was newly synthesized as a sensitizing polysaccharide host to examine the chiroptical properties, supramolecular complexation, and photochirogenic behavior with (Z,Z)-1,3-cyclooctadiene (1ZZ). The enantiodifferentiating photoisomerization of 1ZZ included and sensitized by this polysaccharide host gave a highly strained chiral (E,Z)-isomer in up to 8.7% enantiomeric excess (ee) in solution and 11.7% ee in the solid state, which are the highest values ever reported for a supramolecular photochirogenesis of 1EZ.
@article{fukuhara2011enantiodifferentiating, title = {Enantiodifferentiating photoisomerization of (Z, Z)-1, 3-cyclooctadiene included and sensitized by naphthoyl-curdlan}, author = {Fukuhara, Gaku and Imai, Mami and Yang, Cheng and Mori, Tadashi and Inoue, Yoshihisa}, journal = {Org. Lett.}, volume = {13}, number = {7}, pages = {1856--1859}, year = {2011}, publisher = {ACS Publications}, doi = {10.1021/ol2003644}, url = {https://doi.org/10.1021/ol2003644}, dimensions = {true}, tab = {paper}, } -
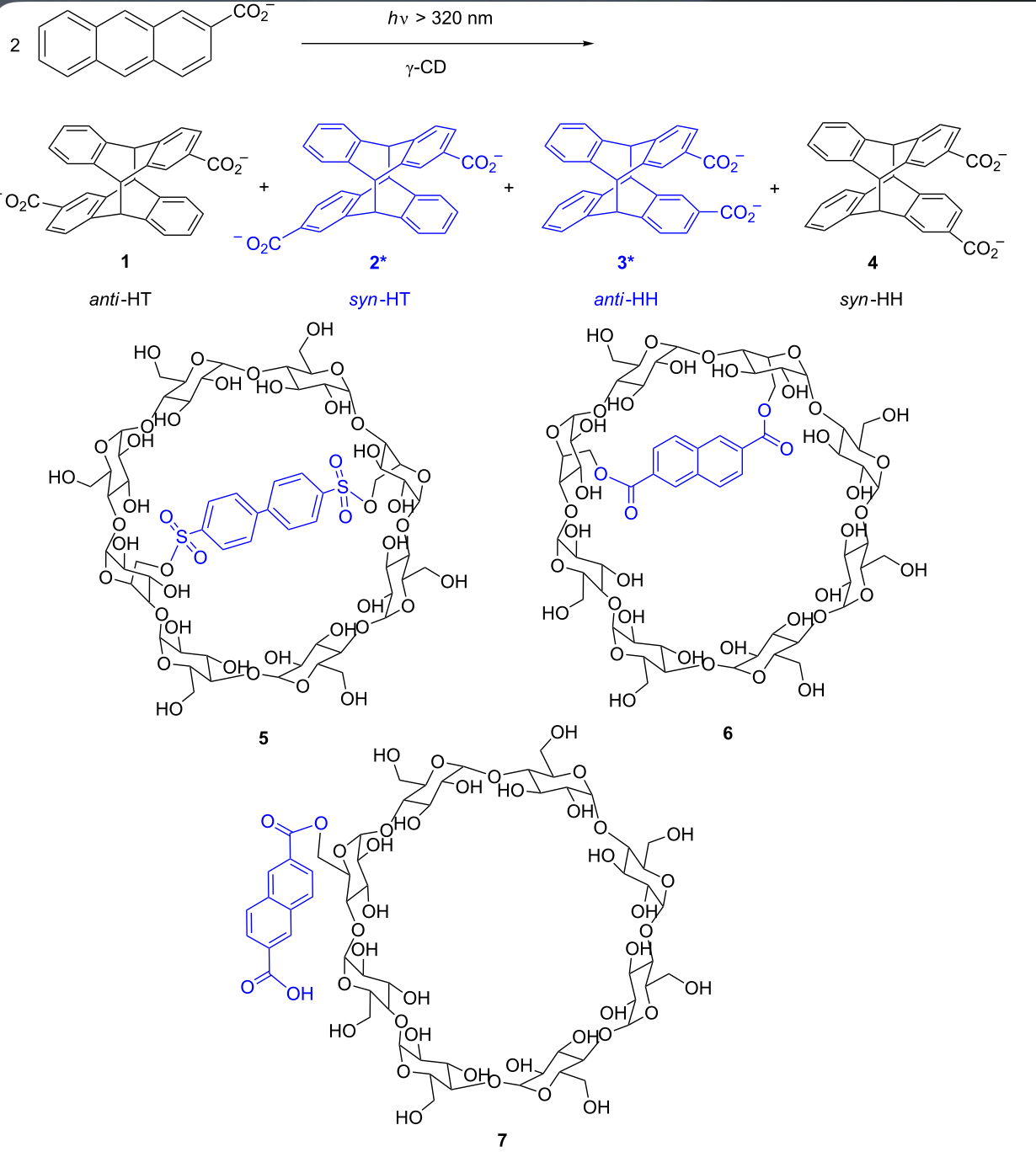 Supramolecular FRET photocyclodimerization of anthracenecarboxylate with naphthalene-capped γ-cyclodextrinQian Wang, Cheng Yang*, Gaku Fukuhara, Tadashi Mori, Yu Liu, and Yoshihisa Inoue*Beilstein J. Org. Chem., 2011, 7, 290–297.
Supramolecular FRET photocyclodimerization of anthracenecarboxylate with naphthalene-capped γ-cyclodextrinQian Wang, Cheng Yang*, Gaku Fukuhara, Tadashi Mori, Yu Liu, and Yoshihisa Inoue*Beilstein J. Org. Chem., 2011, 7, 290–297.γ-Cyclodextrin (CD) derivatives with a naphthalene moiety anchored to one or two of the glucose units of the CD were synthesized in order to investigate the effects of flexible and rigid capping upon complexation, as well as Förster resonance energy transfer (FRET) and photochirogenic behavior of anthracenecarboxylate (AC) moieties. UV–vis, circular dichroism and fluorescence spectral studies revealed that two AC molecules are simultaneously included in the modified γ-CD cavity to form a right-handed screw and also that the naphthalene cap efficiently transfers the singlet energy to AC included in the CD cavity via the FRET mechanism. Compared to native γ-CD, the modified γ-CDs showed much higher first association constants (K1) but relatively lower second association constants (K2) for AC, leading to two-fold larger overall affinities (K1K2). Photocyclodimerization of AC with these modified γ-CDs produced more head-to-head (HH) dimers in much better enantiomeric excesses (ee) for anti-HH dimer compared to native γ-CD. Interestingly, FRET excitation further enhanced the chemical and optical yields of anti-HH dimer up to 36% and 35% ee, for which the highly efficient FRET sensitization within the CD cavity, minimizing the “contamination” from the achiral “outside” photoreaction, is responsible. FRET sensitization also enabled us to achieve the catalytic photocyclodimerization of AC with a sub-equivalent amount of chiral supramolecular host.
@article{wang2011supramolecular, title = {Supramolecular FRET photocyclodimerization of anthracenecarboxylate with naphthalene-capped $\gamma$-cyclodextrin}, author = {Wang, Qian and Yang, Cheng and Fukuhara, Gaku and Mori, Tadashi and Liu, Yu and Inoue, Yoshihisa}, journal = {Beilstein J. Org. Chem.}, volume = {7}, number = {1}, pages = {290--297}, year = {2011}, publisher = {Beilstein-Institut}, doi = {10.3762/bjoc.7.38}, url = {https://doi.org/10.3762/bjoc.7.38}, dimensions = {true}, tab = {paper}, } -
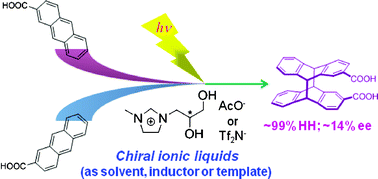 Chiral ionic liquid-mediated photochirogenesis. Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acidGaku Fukuhara*, Takahiro Okazaki, Marco Lessi, Masaki Nishijima, Cheng Yang, Tadashi Mori, Andrea Mele, Fabio Bellina, Cinzia Chiappe*, and Yoshihisa Inoue*Org. Biomol. Chem., 2011, 9, 7105–7112.
Chiral ionic liquid-mediated photochirogenesis. Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acidGaku Fukuhara*, Takahiro Okazaki, Marco Lessi, Masaki Nishijima, Cheng Yang, Tadashi Mori, Andrea Mele, Fabio Bellina, Cinzia Chiappe*, and Yoshihisa Inoue*Org. Biomol. Chem., 2011, 9, 7105–7112.Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid (AC-H) and its lithium salt (AC-Li) in chiral ionic liquid (CIL), (R)-1-(2,3-dihydroxypropyl)-3-methylimidazolium acetate [(R)-GLYMI][AcO], gave a mixture of two head-to-tail (HT) and two head-to-head (HH) cyclodimers in HT/HH ratios of 1.3–1.7 (for AC-H) and 2.2–4.3 (for AC-Li) with low enantiomeric excesses (ee) of 0–3% for chiral syn-HT and anti-HH dimers. In contrast, irradiation of AC-H in an aqueous solution, containing cucurbit[8]uril (CB[8]) as a host and [(R)-GLYMI][AcO] or [(R)-GLYMI][Tf2N] as a modifier of CB portals, afforded the HH dimers in 91–99% selectivity, although the anti-HH dimer was totally racemic. Interestingly, irradiation of AC-H in a dichloromethane solution, containing [(R)-GLYMI][AcO] as a chiral template, led to the formation of the HH-dimers in 98% selectivity with chiral anti-HH dimer in −14% ee, presumably by the dual ligation of two ACs to a CIL through electrostatic and hydrogen-bonding interactions.
@article{fukuhara2011chiral, title = {Chiral ionic liquid-mediated photochirogenesis. Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid}, author = {Fukuhara, Gaku and Okazaki, Takahiro and Lessi, Marco and Nishijima, Masaki and Yang, Cheng and Mori, Tadashi and Mele, Andrea and Bellina, Fabio and Chiappe, Cinzia and Inoue, Yoshihisa}, journal = {Org. Biomol. Chem.}, volume = {9}, number = {20}, pages = {7105--7112}, year = {2011}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c1ob05716b}, url = {https://doi.org/10.1039/c1ob05716b}, dimensions = {true}, tab = {paper}, } -
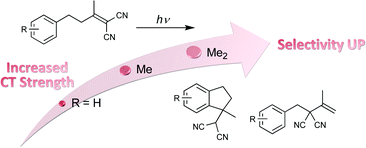 Competitive photocyclization/rearrangement of 4-aryl-1, 1-dicyanobutenes controlled by intramolecular charge-transfer interaction. Effect of medium polarity, temperature, pressure, excitation wavelength, and confinementTadashi Ito, Emi Nishiuchi, Gaku Fukuhara, Yoshihisa Inoue*, and Tadashi Mori*Photochem. Photobiol. Sci., 2011, 10, 1405–1414.
Competitive photocyclization/rearrangement of 4-aryl-1, 1-dicyanobutenes controlled by intramolecular charge-transfer interaction. Effect of medium polarity, temperature, pressure, excitation wavelength, and confinementTadashi Ito, Emi Nishiuchi, Gaku Fukuhara, Yoshihisa Inoue*, and Tadashi Mori*Photochem. Photobiol. Sci., 2011, 10, 1405–1414.A series of 4-aryl-1,1-dicyanobutenes (1a-1f) with different substituents were synthesized to control the intramolecular donor-acceptor or charge-transfer (C-T) interactions in the ground state. Photoexcitation of these C-T substrates led to competitive cyclization and rearrangement, the ratio being critically controlled by various environmental factors, such as solvent polarity, temperature and static pressure, and also by excitation wavelength and supramolecular confinement (polyethylene voids). In non-polar solvents, the rearrangement was dominant (>10: 1) for all examined substrates, while the cyclization was favoured in polar solvents, in particular at low temperatures. Selective excitation at the C-T band further enhanced the cyclization up to >50: 1 ratios. More importantly, the cyclization/rearrangement ratio was revealed to be a linear function of the C-T transition energy. However, the substrates with a sterically demanding or highly electron-donating substituent failed to give the cyclization product.
@article{ito2011competitive, title = {Competitive photocyclization/rearrangement of 4-aryl-1, 1-dicyanobutenes controlled by intramolecular charge-transfer interaction. Effect of medium polarity, temperature, pressure, excitation wavelength, and confinement}, author = {Ito, Tadashi and Nishiuchi, Emi and Fukuhara, Gaku and Inoue, Yoshihisa and Mori, Tadashi}, journal = {Photochem. Photobiol. Sci.}, volume = {10}, number = {9}, pages = {1405--1414}, year = {2011}, publisher = {Springer}, doi = {10.1039/c1pp05038a}, url = {https://doi.org/10.1039/c1pp05038a}, dimensions = {true}, tab = {paper}, } -
 Supramolecular complexation and photocyclodimerization of methyl 3-methoxy-2-naphthoate with modified γ-cyclodextrinsWenting Liang, Hui-Hui Zhang, Jing-Jing Wang, Yuan Peng, Bin Chen, Chen-Ho Yang, Li-Zhu Wu, Gaku Fukuhara, Tadashi Mori, and Yoshihisa InouePure Appl. Chem., 2011, 83, 769–778.
Supramolecular complexation and photocyclodimerization of methyl 3-methoxy-2-naphthoate with modified γ-cyclodextrinsWenting Liang, Hui-Hui Zhang, Jing-Jing Wang, Yuan Peng, Bin Chen, Chen-Ho Yang, Li-Zhu Wu, Gaku Fukuhara, Tadashi Mori, and Yoshihisa InouePure Appl. Chem., 2011, 83, 769–778.A series of modified γ-cyclodextrins (CDs) were repared as chiral host for catalyzing the enantiodifferentiating photocyclodimerization of methyl 3-methoxy-2-naph-thoate (NA). The complexation behavior of NA with modifiedγ-CDs was studied by UV–vis,fluorescence, and circular dichroism spectroscopies. All of the modified γ-CDs formed stable1:2 host–guest complex with NA, and binding affinities for two-step complexation riticallydepended on the structure of modified γ-CDs. The enantioselectivity of NA photocyclodimerization was also significantly affected by the modification of γ-CD. Thesecondary rim-modification considerably reduced the enantioselectivity, for which the inter-rupted hydrogen-bonding network, leading to a flexible CD skeleton, is most probably responsible.
@article{liang2011supramolecular, title = {Supramolecular complexation and photocyclodimerization of methyl 3-methoxy-2-naphthoate with modified γ-cyclodextrins}, author = {Liang, Wenting and Zhang, Hui-Hui and Wang, Jing-Jing and Peng, Yuan and Chen, Bin and Yang, Cheng an Tung, Chen-Ho and Wu, Li-Zhu and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa}, journal = {Pure Appl. Chem.}, volume = {83}, number = {4}, pages = {769--778}, year = {2011}, publisher = {DE Gruyter}, doi = {10.1351/PAC-CON-10-10-04}, url = {https://doi.org/10.1351/PAC-CON-10-10-04}, dimensions = {true}, tab = {paper}, } -
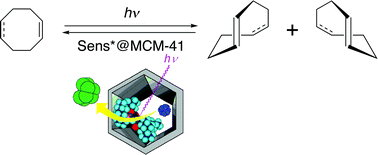 Role of entropy in supramolecular photochirogenesis: Enantiodifferentiating photoisomerization of cyclooctenes in chiral sensitizer-immobilized MCM-41 cavitiesRyo Maeda, Takehiko Wada*, Atsushi Kusaka, Tadashi Mori, Masakazu Iwamoto, and Yoshihisa Inoue*Photochem. Photobiol. Sci., 2011, 10, 1390–1392.
Role of entropy in supramolecular photochirogenesis: Enantiodifferentiating photoisomerization of cyclooctenes in chiral sensitizer-immobilized MCM-41 cavitiesRyo Maeda, Takehiko Wada*, Atsushi Kusaka, Tadashi Mori, Masakazu Iwamoto, and Yoshihisa Inoue*Photochem. Photobiol. Sci., 2011, 10, 1390–1392.To examine the effects of confinement, or low-entropy environments, we employed the mesoporous silicate MCM-41 modified with optically active benzenetetracarboxylates as chiral reaction media to effect the enantiodifferentiating photoisomerization of (Z)-cyclooctene and (Z,Z)-1,5-cyclooctadiene. The ee of the (E)-isomer produced and its temperature dependence behavior in MCM-41 were completely different from those observed in homogeneous solutions. Immobilizing the sensitizer in MCM-41 reduced the contribution of the entropy factor.
@article{maeda2011role, title = {Role of entropy in supramolecular photochirogenesis: Enantiodifferentiating photoisomerization of cyclooctenes in chiral sensitizer-immobilized MCM-41 cavities}, author = {Maeda, Ryo and Wada, Takehiko and Kusaka, Atsushi and Mori, Tadashi and Iwamoto, Masakazu and Inoue, Yoshihisa}, journal = {Photochem. Photobiol. Sci.}, volume = {10}, number = {9}, pages = {1390--1392}, year = {2011}, publisher = {Springer}, doi = {10.1039/c1pp05087g}, url = {https://doi.org/10.1039/c1pp05087g}, dimensions = {true}, tab = {paper}, } -
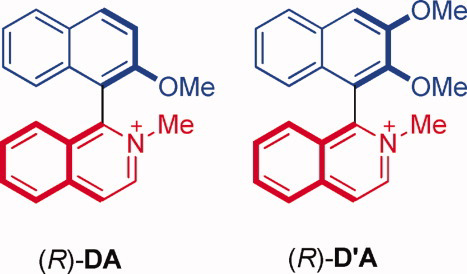 Experimental and theoretical investigations of circular dichroism of donor–acceptor 1, 1′-binaphthyls: Influence of substitution on the coupling amplitude and cotton effect of the charge-transfer bandYoshito Nakai, Masaki Nishizaka, Cheng Yang, Gaku Fukuhara, Tadashi Mori*, and Yoshihisa InoueChirality, 2011, 23, E22–E27.
Experimental and theoretical investigations of circular dichroism of donor–acceptor 1, 1′-binaphthyls: Influence of substitution on the coupling amplitude and cotton effect of the charge-transfer bandYoshito Nakai, Masaki Nishizaka, Cheng Yang, Gaku Fukuhara, Tadashi Mori*, and Yoshihisa InoueChirality, 2011, 23, E22–E27.The electronic circular dichroism (CD) spectra of donor–acceptor binaphthyls were investigated experimentally and theoretically. The enantiomerically pure forms of 1-(2-methoxy-1-naphthyl)- and 1-(2,3-dimethoxy-1-naphthyl)-2-methylisoquinolinium tetrafluoroborates (DA and D′A) were prepared, and their UV–vis and CD spectra were compared. The donor–acceptor interaction was apparent from the absorption at longer wavelengths, whereas its strength was not very different from each other. In addition, very similar structures were obtained for the two aromatic planes in DA and D′A when the geometry was optimized by the density functional theory. The additional methoxy group in the latter spices scarcely disturbed the UV–vis spectrum but significantly affected the CD spectrum. Thus, the observed CD spectra were considerably different from each other, especially in the 1Bb band couplet, where the amplitude was reduced to almost one-fourth in D′A. The theoretical investigations led to the following conclusions: (1) The potential curve associated with the central CC dihedral angle of 1,1′-binaphthyl is fairly flat at the bottom for both DA and D′A and freely rotating at an ambient temperature. The potential curve of D′A is, however, significantly different from that of DA, in which the curve is much steeper and biased to the s-cis side. As the observed CD spectrum is an ensemble of conformers of various dihedral angles, such difference in potential certainly affects the overall spectrum; (2) The additional methoxy group introduced at the 3-position effectively altered the CD spectral pattern, which was theoretically supported by the calculation at the RI-CC2/TZVPP level; (3) Consequently, the classical coupled oscillator theory, in which the angle between the transition dipole moments of two aromatic planes is solely considered, is not applicable to the quantitative evaluation of the chiroptical properties of 1,1′-binaphthyls; rather, the quantum chemical approach is preferred, permitting a direct comparison with the experiment.
@article{nakai2011experimental, title = {Experimental and theoretical investigations of circular dichroism of donor--acceptor 1, 1′-binaphthyls: Influence of substitution on the coupling amplitude and cotton effect of the charge-transfer band}, author = {Nakai, Yoshito and Nishizaka, Masaki and Yang, Cheng and Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa}, journal = {Chirality}, volume = {23}, number = {1E}, pages = {E22--E27}, year = {2011}, publisher = {Wiley Online Library}, doi = {10.1002/chir.20947}, url = {https://doi.org/10.1002/chir.20947}, dimensions = {true}, tab = {paper}, } -
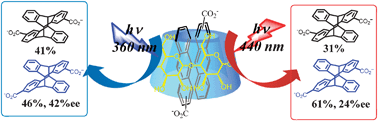 Wavelength-controlled supramolecular photocyclodimerization of anthracenecarboxylate mediated by γ-cyclodextrinsQian Wang, Cheng Yang*, Chengfeng Ke, Gaku Fukuhara, Tadashi Mori, Yu Liu*, and Yoshihisa Inoue*Chem. Commun., 2011, 47, 6849–6851.
Wavelength-controlled supramolecular photocyclodimerization of anthracenecarboxylate mediated by γ-cyclodextrinsQian Wang, Cheng Yang*, Chengfeng Ke, Gaku Fukuhara, Tadashi Mori, Yu Liu*, and Yoshihisa Inoue*Chem. Commun., 2011, 47, 6849–6851.Stereochemical outcomes were critically tuned by excitation wavelength in the supramolecular photocyclodimerization of 2-anthracenecarboxylic acid mediated by native and diamino-modified γ-cyclodextrins.
@article{wang2011wavelength, title = {Wavelength-controlled supramolecular photocyclodimerization of anthracenecarboxylate mediated by γ-cyclodextrins}, author = {Wang, Qian and Yang, Cheng and Ke, Chengfeng and Fukuhara, Gaku and Mori, Tadashi and Liu, Yu and Inoue, Yoshihisa}, journal = {Chem. Commun.}, volume = {47}, number = {24}, pages = {6849--6851}, year = {2011}, publisher = {Royal Society of Chemistry}, doi = {10.1039/c1cc11771h}, url = {https://doi.org/10.1039/c1cc11771h}, dimensions = {true}, tab = {paper}, } -
 Introduction to the themed issue in honour of the contribution of Japanese scientists to photochemistryCornelia Bohne and Tadashi MoriPhotochem. Photobiol. Sci., 2011, 10, 1379–1379.
Introduction to the themed issue in honour of the contribution of Japanese scientists to photochemistryCornelia Bohne and Tadashi MoriPhotochem. Photobiol. Sci., 2011, 10, 1379–1379.A graphical abstract is available for this content
@article{bohne2011introduction, title = {Introduction to the themed issue in honour of the contribution of Japanese scientists to photochemistry}, author = {Bohne, Cornelia and Mori, Tadashi}, journal = {Photochem. Photobiol. Sci.}, volume = {10}, number = {9}, pages = {1379--1379}, year = {2011}, publisher = {Springer}, doi = {10.1039/c1pp90023d}, url = {https://doi.org/10.1039/c1pp90023d}, dimensions = {true}, tab = {paper}, }