articles in 2009
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
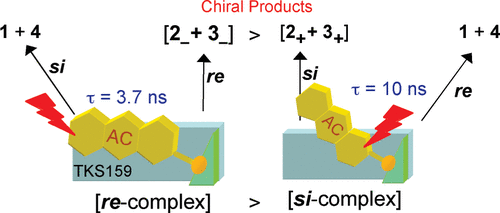 Supramolecular complexation and enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid with 4-aminoprolinol derivatives as chiral hydrogen-bonding templatesYuko Kawanami, Tamara CS Pace, Jun-ichi Mizoguchi, Toshiharu Yanagi, Masaki Nishijima, Tadashi Mori, Takehiko Wada, Cornelia Bohne*, and Yoshihisa Inoue*J. Org. Chem., 2009, 74, 7908–7921.
Supramolecular complexation and enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid with 4-aminoprolinol derivatives as chiral hydrogen-bonding templatesYuko Kawanami, Tamara CS Pace, Jun-ichi Mizoguchi, Toshiharu Yanagi, Masaki Nishijima, Tadashi Mori, Takehiko Wada, Cornelia Bohne*, and Yoshihisa Inoue*J. Org. Chem., 2009, 74, 7908–7921.The photochirogenesis of 2-anthracenecarboxylic acid (AC) complexed to a hydrogen-bonding template (TKS159) was investigated to obtain mechanistic information on how chirogenesis is achieved for the dimerization of AC. Complexation of AC to TKS159 leads to the shielding of one of the two surfaces of the prochiral AC molecule. The two diastereomeric AC−TKS complexes, i.e., re-AC−TKS and si-AC−TKS, were characterized by changes in the UV−vis, fluorescence, and circular dichroism spectra and excited-state lifetimes. The ee is not simply determined by the diastereomeric ratio of the re- and si-AC−TKS complexes but also depends on the relative lifetimes of the diastereomeric complexes. The relative population of the re and si complexes was calculated from the enantiomeric excess (ee) for the products, taking into account the relative lifetimes of the two complexes. These studies established a protocol that can be used to reveal the mechanism for photochirogenesis by investigating the ground state and the excited state behavior of supramolecular systems.
@article{kawanami2009supramolecular, title = {Supramolecular complexation and enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid with 4-aminoprolinol derivatives as chiral hydrogen-bonding templates}, author = {Kawanami, Yuko and Pace, Tamara CS and Mizoguchi, Jun-ichi and Yanagi, Toshiharu and Nishijima, Masaki and Mori, Tadashi and Wada, Takehiko and Bohne, Cornelia and Inoue, Yoshihisa}, journal = {J. Org. Chem.}, volume = {74}, number = {20}, pages = {7908--7921}, year = {2009}, publisher = {ACS Publications}, doi = {10.1021/jo901792t}, url = {https://doi.org/10.1021/jo901792t}, dimensions = {true}, tab = {paper}, } -
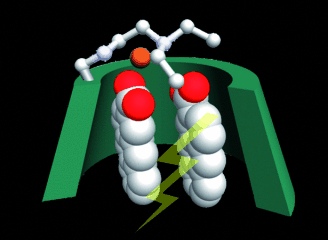 Catalytic enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by a non-sensitizing chiral metallosupramolecular hostChenfeng Ke, Cheng Yang, Tadashi Mori, Takehiko Wada, Yu Liu*, and Yoshihisa Inoue*Angew. Chem. Int. Ed., 2009, 121, 6803–6805.
Catalytic enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by a non-sensitizing chiral metallosupramolecular hostChenfeng Ke, Cheng Yang, Tadashi Mori, Takehiko Wada, Yu Liu*, and Yoshihisa Inoue*Angew. Chem. Int. Ed., 2009, 121, 6803–6805.Combined use of diamino-γ-cyclodextrin (CD) and Cu(ClO4)2 resulted in the first catalytic supramolecular photochirogenesis in the photocyclodimerization of 2-anthracenecarboxylic acid. The anti-head-to-head cyclodimer formed in 64–70% enantiomeric excess and about 50% yield; these values are the highest ever reported for CD-mediated photochirogenesis.
@article{ke2009catalytic, title = {Catalytic enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by a non-sensitizing chiral metallosupramolecular host}, author = {Ke, Chenfeng and Yang, Cheng and Mori, Tadashi and Wada, Takehiko and Liu, Yu and Inoue, Yoshihisa}, journal = {Angew. Chem. Int. Ed.}, volume = {121}, number = {36}, pages = {6803--6805}, year = {2009}, publisher = {WILEY-VCH Verlag Weinheim}, doi = {10.1002/anie.200902911}, url = {https://doi.org/10.1002/anie.200902911}, dimensions = {true}, tab = {paper}, } -
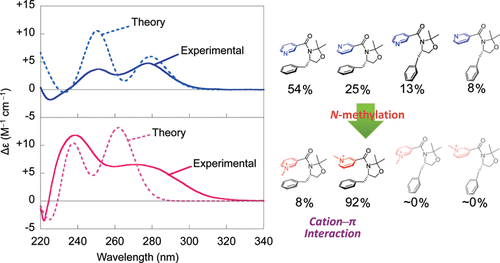 Combined experimental and quantum chemical investigation of chiroptical properties of nicotinamide derivatives with and without intramolecular cation- \pi interactionsAkinori Shimizu, Tadashi Mori*, Yoshihisa Inoue*, and Shinji YamadaJ. Phys. Chem. A, 2009, 113, 8754–8764.
Combined experimental and quantum chemical investigation of chiroptical properties of nicotinamide derivatives with and without intramolecular cation- \pi interactionsAkinori Shimizu, Tadashi Mori*, Yoshihisa Inoue*, and Shinji YamadaJ. Phys. Chem. A, 2009, 113, 8754–8764.The circular dichroism (CD) spectra of neutral and cationic nicotinamide derivatives were experimentally examined in solution and in the solid state to show dramatic differences in the two phases and appreciable dependence on temperature and solvent. The CD spectrum of neutral nicotinamide 1 in solution was reproduced theoretically by averaging the theoretical spectra calculated for all of the extended and folded conformers (s-trans-G+, s-cis-G+, s-trans-T, and s-cis-T) weighted by their population. The preference for the folded, over the extended, conformers in less polar solvent was indicated by the calculation and confirmed experimentally by the analysis of specific rotations. Theoretical CD spectrum calculated for the conformer found in the X-ray structural analysis (s-cis-T) well reproduced the experimental CD spectrum measured in the solid state. Introducing cation−π interactions by N-methylation of 1 to give 1-Me+ led to dramatic changes in CD spectrum. Nevertheless, the experimental CD spectrum of 1-Me+ was well reproduced by averaging the theoretical spectra calculated for a pair of most stable conformers (s-cis-G+ and s-trans-G+) of 1-Me+. The CD spectrum calculated for the s-trans-G+ conformer, which was found in the X-ray crystallographic analysis, did not agree with the experimental one. The theoretical spectra were better reproduced in general by the more sophisticated RI-CC2 method, but the conventional TD-DFT method also gave acceptable results. This allowed us to successfully calculate the larger derivative 2-Me+, for which the RI-CC2 method was not practically applicable. These results show that the structure/conformation may vary with the conditions employed (e.g., by altering the solvent or phase) and thus the experimental analysis under the identical condition is essential for a serious structural study. The present study on a series of nicotinamide derivatives 1, 1-Me+, and 2-Me+ with and without cation−π interactions demonstrates that the combination of experimental and theoretical chiroptical methods is capable of providing reliable structural/conformational information in solution phase, which is complementary to the X-ray crystallographic structure in the solid state.
@article{shimizu2009combined, title = {Combined experimental and quantum chemical investigation of chiroptical properties of nicotinamide derivatives with and without intramolecular cation- $\pi$ interactions}, author = {Shimizu, Akinori and Mori, Tadashi and Inoue, Yoshihisa and Yamada, Shinji}, journal = {J. Phys. Chem. A}, volume = {113}, number = {30}, pages = {8754--8764}, year = {2009}, publisher = {ACS Publications}, doi = {10.1021/jp904243w}, url = {https://doi.org/10.1021/jp904243w}, dimensions = {true}, tab = {paper}, } -
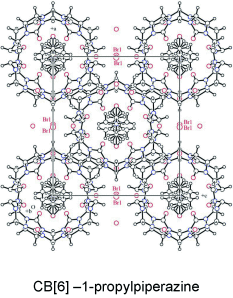 Supramolecular Complexation of N-Alkyl-and N, N′-Dialkylpiperazines with Cucurbit [6] uril in Aqueous Solution and in the Solid StateMikhail V Rekharsky, Hatsuo Yamamura, Tadashi Mori, Akihiro Sato, Motoo Shiro, Sergey V Lindeman, Rajendra Rathore, Kouhei Shiba, Young Ho Ko, Narayanan Selvapalam, Kimoon Kim*, and Yoshihisa Inoue*Chem. Eur. J., 2009, 15, 1957–1965.
Supramolecular Complexation of N-Alkyl-and N, N′-Dialkylpiperazines with Cucurbit [6] uril in Aqueous Solution and in the Solid StateMikhail V Rekharsky, Hatsuo Yamamura, Tadashi Mori, Akihiro Sato, Motoo Shiro, Sergey V Lindeman, Rajendra Rathore, Kouhei Shiba, Young Ho Ko, Narayanan Selvapalam, Kimoon Kim*, and Yoshihisa Inoue*Chem. Eur. J., 2009, 15, 1957–1965.Complex stoichiometry/composition and degree of oligomerization (oligomeric supramolecular complex formation) of cucurbit[6]uril (CB[6]) with N-alkyl- and N,N′-dialkylpiperazine were investigated in aqueous solutions by means of isothermal titration calorimetry (ITC), ESI-MS, NMR and light scattering measurements. It was found that the complex stability and the degree of oligomerization increase with elongating the alkyl chain attached to the piperazine core. X-ray crystallographic studies revealed a clear correlation between the structure of CB[6]–alkylpiperazine crystals obtained from aqueous solutions and the molecular weight/properties of host–guest oligomers existed in the solution as supramolecular “seeds” of crystal formation.
@article{rekharsky2009supramolecular, title = {Supramolecular Complexation of N-Alkyl-and N, N′-Dialkylpiperazines with Cucurbit [6] uril in Aqueous Solution and in the Solid State}, author = {Rekharsky, Mikhail V and Yamamura, Hatsuo and Mori, Tadashi and Sato, Akihiro and Shiro, Motoo and Lindeman, Sergey V and Rathore, Rajendra and Shiba, Kouhei and Ko, Young Ho and Selvapalam, Narayanan and Kim, Kimoon and Inoue, Yoshihisa}, journal = {Chem. Eur. J.}, volume = {15}, number = {8}, pages = {1957--1965}, year = {2009}, publisher = {Wiley Online Library}, doi = {10.1002/chem.200800398}, url = {https://doi.org/10.1002/chem.200800398}, dimensions = {true}, tab = {paper}, } -
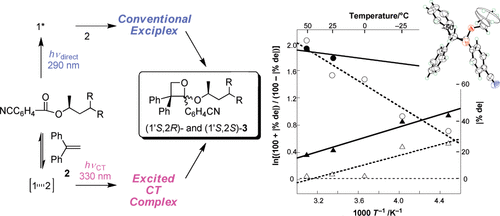 Wavelength Control of Diastereodifferentiating Paternò- Büchi Reaction of Chiral Cyanobenzoates with Diphenylethene through Direct versus Charge-Transfer ExcitationKazuyuki Matsumura, Tadashi Mori, and Yoshihisa InoueJ. Am. Chem. Soc., 2009, 131, 17076–17077.
Wavelength Control of Diastereodifferentiating Paternò- Büchi Reaction of Chiral Cyanobenzoates with Diphenylethene through Direct versus Charge-Transfer ExcitationKazuyuki Matsumura, Tadashi Mori, and Yoshihisa InoueJ. Am. Chem. Soc., 2009, 131, 17076–17077.In the diastereodifferentiating Paternó−Büchi reaction, the excited CT complex was distinctly different in structure and reactivity from the conventional exciplex, and the inherent diastereofacial selectivity and its temperature dependence were opposite to each other in these two excitation modes. Thus, the combined use of wavelength and temperature not only reveals the mechanistic details but also provides a new convenient, powerful tool for critically controlling the stereochemical outcomes of asymmetric photoreactions.
@article{matsumura2009wavelength, title = {Wavelength Control of Diastereodifferentiating Paternò- Büchi Reaction of Chiral Cyanobenzoates with Diphenylethene through Direct versus Charge-Transfer Excitation}, author = {Matsumura, Kazuyuki and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Am. Chem. Soc.}, volume = {131}, number = {47}, pages = {17076--17077}, year = {2009}, publisher = {ACS Publications}, doi = {10.1021/ja907156j}, url = {https://doi.org/10.1021/ja907156j}, dimensions = {true}, tab = {paper}, } -
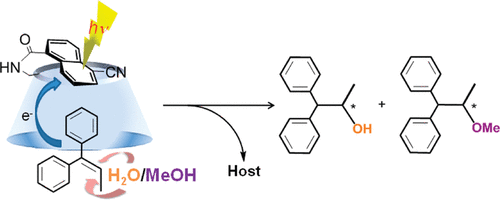 Competitive enantiodifferentiating anti-Markovnikov photoaddition of water and methanol to 1, 1-diphenylpropene using a sensitizing cyclodextrin hostGaku Fukuhara, Tadashi Mori, and Yoshihisa Inoue*J. Org. Chem., 2009, 74, 6714–6727.
Competitive enantiodifferentiating anti-Markovnikov photoaddition of water and methanol to 1, 1-diphenylpropene using a sensitizing cyclodextrin hostGaku Fukuhara, Tadashi Mori, and Yoshihisa Inoue*J. Org. Chem., 2009, 74, 6714–6727.UV−vis, circular dichroism (CD), fluorescence, and NMR spectral studies on the self-inclusion behavior of a newly synthesized sensitizing host, 6-(5-cyanonaphthyl-1-carboamido)-6-deoxy-β-cyclodextrin (1), showed that the appended naphthalene moiety of 1 perches laterally on the cyclodextrin rim in aqueous methanol but is shallowly included and somewhat tilted in its own cavity in water. UV−vis and CD spectral examinations of the complexation of guest substrate 1,1-diphenylpropene (DPP) with host 1 revealed the formation of a stoichiometric 1:1 complex of DPP with 1. The naphthyl fluorescence of 1 was efficiently quenched by the addition of DPP in aqueous solutions of low methanol contents (≤25%) but was less efficiently quenched in more hydrophobic solvents (≥50% methanol), where the fluorophore is not included in the cavity and allows the external attack of DPP to form an exciplex in the bulk solution. Upon irradiation in aqueous solutions of different methanol contents, competitive photoaddition of water and methanol to DPP occurred to give chiral water adduct 3 and methanol adduct 4, favoring the latter product by a factor of 2.5 due to the higher nucleophilicity of methanol. The enantiomeric excess (ee) values of the photoadducts were generally low in highly methanolic solutions, but was greatly improved by increasing the water content to reach 18% ee for 3 and 13% ee for 4 in 10% methanol solution at −10 °C. Interestingly, the ee of methanol adduct 4 was consistently lower than that of water adduct 3 particularly in water-rich solvents, revealing that the product’s ee is not a simple thermodynamic function of the enantioface-selectivity upon complexation of DPP by chiral host 1 but also kinetically controlled by the subsequent photoinduced enantioface-differentiating nucleophilic attack of water and methanol to radical cationic DPP generated photochemically. Compatible with this mechanism, the compensation plot of the differential activation enthalpy versus entropy, which were obtained from the van’t Hoff analysis of the temperature-dependent ee’s obtained in aqueous solutions of varying methanol contents, gave an excellent straight line for water adduct 3 but an unprecedented bent plot for methanol adduct 4, indicating a switching of the mechanism in between 35 and 50% methanol solution. By using high pressure, low temperature, and/or added salt, the ee of water adduct 3 was further enhanced to 24−26%.
@article{fukuhara2009competitive, title = {Competitive enantiodifferentiating anti-Markovnikov photoaddition of water and methanol to 1, 1-diphenylpropene using a sensitizing cyclodextrin host}, author = {Fukuhara, Gaku and Mori, Tadashi and Inoue, Yoshihisa}, journal = {J. Org. Chem.}, volume = {74}, number = {17}, pages = {6714--6727}, year = {2009}, publisher = {ACS Publications}, doi = {10.1021/jo9012628}, url = {https://doi.org/10.1021/jo9012628}, dimensions = {true}, tab = {paper}, }