articles in 2007
all / 2026 / 2025 / 2024 / 2023 / 2022 / 2021 / 2020 / 2019 / 2018 / 2017 / 2016 / 2015 / 2014 / 2013 / 2012 / 2011 / 2010 / 2009 / 2008 / 2007 / 2006 / 2005 / 2004 / 2003 / 2002 / 2001 / 2000 / 1999 / 1998 / 1997 / 1996 / 1995 / 1994 / 1993
-
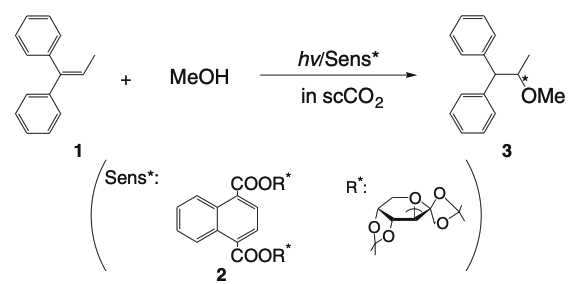 Critical control by temperature and pressure of enantiodifferentiating anti-Markovnikov photoaddition of methanol to diphenylpropene in near critical and supercritical carbon dioxideYasuhiro Nishiyama, Takehiko Wada, Tadashi Mori, and Yoshihisa Inoue*Chem. Lett., 2007, 36, 1488–1489.
Critical control by temperature and pressure of enantiodifferentiating anti-Markovnikov photoaddition of methanol to diphenylpropene in near critical and supercritical carbon dioxideYasuhiro Nishiyama, Takehiko Wada, Tadashi Mori, and Yoshihisa Inoue*Chem. Lett., 2007, 36, 1488–1489.The enantiomeric excess of photoadduct obtained in the title reaction was critically manipulated not only by pressure (P) but also by temperature (T), exhibiting substantially different P-dependence profiles at different T, yet sharing a sudden leap near the critical density at each T, for which the difference in clustering property of methanol in near and supercritical CO2 is thought to be responsible.
@article{nishiyama2007critical, title = {Critical control by temperature and pressure of enantiodifferentiating anti-Markovnikov photoaddition of methanol to diphenylpropene in near critical and supercritical carbon dioxide}, author = {Nishiyama, Yasuhiro and Wada, Takehiko and Mori, Tadashi and Inoue, Yoshihisa}, journal = {Chem. Lett.}, volume = {36}, number = {12}, pages = {1488--1489}, year = {2007}, publisher = {Oxford University Press}, doi = {10.1246/cl.2007.1488}, url = {https://doi.org/10.1246/cl.2007.1488}, dimensions = {true}, tab = {paper}, } -
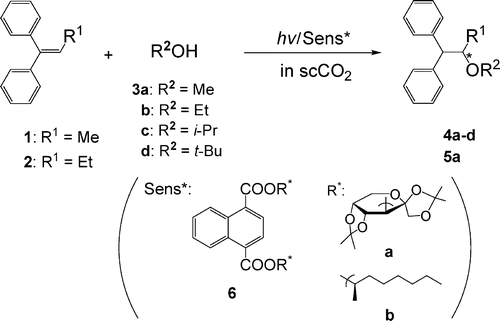 Mechanistic study on the enantiodifferentiating anti-Markovnikov photoaddition of alcohols to 1, 1-diphenyl-1-alkenes in near-critical and supercritical carbon dioxideYasuhiro Nishiyama, Masayuki Kaneda, Sadayuki Asaoka, Ryota Saito, Tadashi Mori, Takehiko Wada, and Inoue* YoshihisaJ. Phys. Chem. A, 2007, 111, 13432–13440.
Mechanistic study on the enantiodifferentiating anti-Markovnikov photoaddition of alcohols to 1, 1-diphenyl-1-alkenes in near-critical and supercritical carbon dioxideYasuhiro Nishiyama, Masayuki Kaneda, Sadayuki Asaoka, Ryota Saito, Tadashi Mori, Takehiko Wada, and Inoue* YoshihisaJ. Phys. Chem. A, 2007, 111, 13432–13440.Enantiodifferentiating anti-Markovnikov photoaddition of alcohol (methanol, ethanol, 2-propanol, and tert-butanol) to aromatic alkene (1,1-diphenylpropene and 1,1-diphenyl-1-butene), sensitized by optically active alkyl and saccharide naphthalene(di)carboxylates, was investigated in supercritical carbon dioxide at varying pressures to elucidate the effects of clustering on photosensitization and enantiodifferentiation behavior, in particular on the product’s enantiomeric excess (ee). For all the alkene/alcohol/chiral sensitizer combinations examined, a sudden change in the product’s ee was consistently observed near the critical density, which is attributable to the critical pressure dependence of clustering around the intervening exciplex intermediate.
@article{nishiyama2007mechanistic, title = {Mechanistic study on the enantiodifferentiating anti-Markovnikov photoaddition of alcohols to 1, 1-diphenyl-1-alkenes in near-critical and supercritical carbon dioxide}, author = {Nishiyama, Yasuhiro and Kaneda, Masayuki and Asaoka, Sadayuki and Saito, Ryota and Mori, Tadashi and Wada, Takehiko and Yoshihisa, Inoue}, journal = {J. Phys. Chem. A}, volume = {111}, number = {51}, pages = {13432--13440}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/jp076179i}, url = {https://doi.org/10.1021/jp076179i}, dimensions = {true}, tab = {paper}, } -
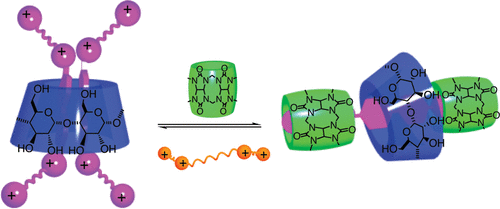 Dynamic switching between single-and double-axial rotaxanes manipulated by charge and bulkiness of axle terminiCheng Yang, Young Ho Ko, Narayanan Selvapalam, Yumi Origane, Tadashi Mori, Takehiko Wada, Kimoon Kim*, and Yoshihisa Inoue*Org. Lett., 2007, 9, 4789–4792.
Dynamic switching between single-and double-axial rotaxanes manipulated by charge and bulkiness of axle terminiCheng Yang, Young Ho Ko, Narayanan Selvapalam, Yumi Origane, Tadashi Mori, Takehiko Wada, Kimoon Kim*, and Yoshihisa Inoue*Org. Lett., 2007, 9, 4789–4792.Twin-axial [3]pseudorotaxanes, in which two multicharged axles simultaneously thread through the γ-CD cavity, were formed for the first time in solution. The twin-axial [3]pseudorotaxane was converted exclusively to a CB [6]-stoppered [4]pseudorotaxane by the addition of CB[6] but regenerated from the [4]pseudorotaxane by the addition of spermine, implementing an unprecedented switching of single/twin-axial rotaxanation.
@article{yang2007dynamic, title = {Dynamic switching between single-and double-axial rotaxanes manipulated by charge and bulkiness of axle termini}, author = {Yang, Cheng and Ko, Young Ho and Selvapalam, Narayanan and Origane, Yumi and Mori, Tadashi and Wada, Takehiko and Kim, Kimoon and Inoue, Yoshihisa}, journal = {Org. Lett.}, volume = {9}, number = {23}, pages = {4789--4792}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/ol702142j}, url = {https://doi.org/10.1021/ol702142j}, dimensions = {true}, tab = {paper}, } -
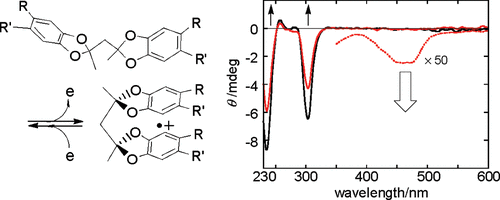 A new class of chiroptical molecular switches based on the redox-induced conformational changesMasato Fukui, Tadashi Mori*, Yoshihisa Inoue, and Rajendra Rathore*Org. Lett., 2007, 9, 3977–3980.
A new class of chiroptical molecular switches based on the redox-induced conformational changesMasato Fukui, Tadashi Mori*, Yoshihisa Inoue, and Rajendra Rathore*Org. Lett., 2007, 9, 3977–3980.A series of optically active bis(catecholketal)s 1−3 were prepared, and their chiroptical properties were investigated experimentally and theoretically, demonstrating that they undergo conformational changes upon 1-e- oxidation and can be used as redox-responsive chiroptical molecular switches.
@article{fukui2007new, title = {A new class of chiroptical molecular switches based on the redox-induced conformational changes}, author = {Fukui, Masato and Mori, Tadashi and Inoue, Yoshihisa and Rathore, Rajendra}, journal = {Org. Lett.}, volume = {9}, number = {20}, pages = {3977--3980}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/ol701639u}, url = {https://doi.org/10.1021/ol701639u}, dimensions = {true}, tab = {paper}, } -
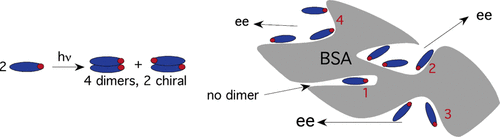 Supramolecular photochirogenesis with biomolecules. Mechanistic studies on the enantiodifferentiation for the photocyclodimerization of 2-anthracenecarboxylate mediated by bovine serum albuminMasaki Nishijima, Tamara CS Pace, Asao Nakamura, Tadashi Mori, Takehiko Wada, Cornelia Bohne*, and Yoshihisa Inoue*J. Org. Chem., 2007, 72, 2707–2715.
Supramolecular photochirogenesis with biomolecules. Mechanistic studies on the enantiodifferentiation for the photocyclodimerization of 2-anthracenecarboxylate mediated by bovine serum albuminMasaki Nishijima, Tamara CS Pace, Asao Nakamura, Tadashi Mori, Takehiko Wada, Cornelia Bohne*, and Yoshihisa Inoue*J. Org. Chem., 2007, 72, 2707–2715.Photophysics and photochemistry of 2-anthracenecarboxylate (AC) bound to bovine serum albumin (BSA) were investigated in detail for the first time by electronic absorption, circular dichroism (CD), steady-state and time-resolved fluorescence, fluorescence quenching, and product analysis studies. Through the spectroscopic investigations, it was revealed that the four independent binding pockets of BSA, which are known to accommodate 1, 3, 2, and 3 AC molecules in the order of decreasing affinity, are distinctly different in hydrophobicity, chiral environment, and accessibility. Interestingly, AC bound to site 1 gave highly structured fluorescence with dual lifetimes of 4.8 and 2.1 ns in an intensity ratio of 3:2, which may be assigned to the existence of two positional or orientational isomers within the very hydrophobic site 1. In contrast, the lifetime of AC in site 2 was much longer (13.3 ns), and ACs in sites 3 and 4 have broader fluorescence spectra with lifetimes that were practically indistinguishable from that in bulk water (15.8 ns). Although each of sites 2−4 simultaneously binds multiple AC molecules, no CD exciton coupling or static fluorescence quenching was detected, indicating that ACs bound to each site are not in close proximity to each other. Quenching studies with nitromethane further confirmed the significant difference in accessibility among the binding sites; thus, ACs bound to sites 1 and 2 are highly protected from the attack of the quencher, affording 32 and 10 times smaller rate constants than that for free AC in water. Product studies in the presence and absence of nitromethane more clearly revealed the photochirogenic performance of each binding site. Although the addition of nitromethane did not greatly alter the product distribution, the enantiomeric excesses (ee’s) of chiral cycloadducts 2 and 3 were critically manipulated by selectively retarding the photoreaction occurring at the more accessible binding sites. Thus, the highest ee of 38% was obtained for 2 in the presence of 18 mM nitromethane, while the highest ee of 58% was attained for 3 in the absence of nitromethane, both at [AC]/[BSA] = 3.6.
@article{nishijima2007supramolecular, title = {Supramolecular photochirogenesis with biomolecules. Mechanistic studies on the enantiodifferentiation for the photocyclodimerization of 2-anthracenecarboxylate mediated by bovine serum albumin}, author = {Nishijima, Masaki and Pace, Tamara CS and Nakamura, Asao and Mori, Tadashi and Wada, Takehiko and Bohne, Cornelia and Inoue, Yoshihisa}, journal = {J. Org. Chem.}, volume = {72}, number = {8}, pages = {2707--2715}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/jo062226b}, url = {https://doi.org/10.1021/jo062226b}, dimensions = {true}, tab = {paper}, } -
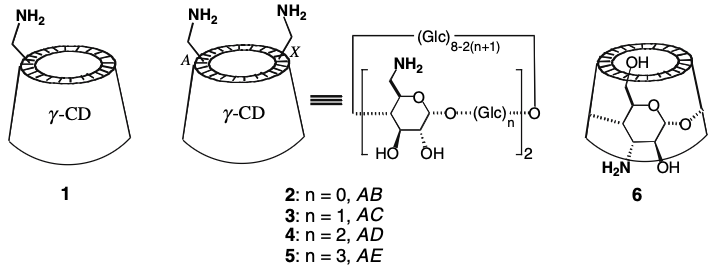 Enhanced ternary 1: 2 host–guest complexation of amino-γ-cyclodextrins with 2-anthracenecarboxylic acidCheng Yang, Gaku Fukuhara, Asao Nakamura, Yumi Origane, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*J. Incl. Phenom. Macrocycl. Chem., 2007, 57, 433–437.
Enhanced ternary 1: 2 host–guest complexation of amino-γ-cyclodextrins with 2-anthracenecarboxylic acidCheng Yang, Gaku Fukuhara, Asao Nakamura, Yumi Origane, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*J. Incl. Phenom. Macrocycl. Chem., 2007, 57, 433–437.The complexation behavior of 6-amino-6-deoxy-γ-cyclodextrin (CD), 6A,6X-diamino-6A,6X-deoxy-γ-CDs and 3A-amino-3A-deoxy-altro-γ-CD with 2-anthracenecarboxylic acid (AC) was studied by NMR, UV–vis and circular dichroism spectroscopy. These modified γ-CD derivatives were found to form stable 1:2 host-guest ternary complexes with AC in aqueous solution. Compared with native γ-CD, the primary-face-aminated γ-CDs exhibited remarkably enhanced overall association constants as a result of the additional electrostatic interactions between the oppositely charged host and guest. In contrast, the ternary complex formation of the secondary-face-aminated γ-CD with AC was hindered.
@article{yang2007enhanced, title = {Enhanced ternary 1: 2 host--guest complexation of amino-$\gamma$-cyclodextrins with 2-anthracenecarboxylic acid}, author = {Yang, Cheng and Fukuhara, Gaku and Nakamura, Asao and Origane, Yumi and Mori, Tadashi and Wada, Takehiko and Inoue, Yoshihisa}, journal = {J. Incl. Phenom. Macrocycl. Chem.}, volume = {57}, number = {1}, pages = {433--437}, year = {2007}, publisher = {Springer}, doi = {10.1007/s10847-006-9230-y}, url = {https://doi.org/10.1007/s10847-006-9230-y}, dimensions = {true}, tab = {paper}, } -
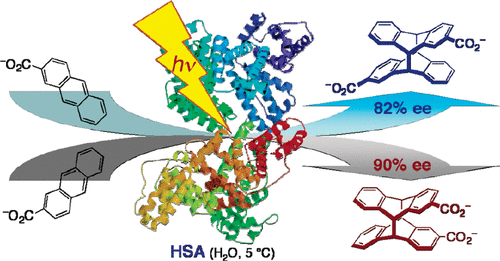 Highly enantiomeric supramolecular [4+ 4] photocyclodimerization of 2-anthracenecarboxylate mediated by human serum albuminMasaki Nishijima, Takehiko Wada, Tadashi Mori, Tamara CS Pace, Cornelia Bohne*, and Yoshihisa Inoue*J. Am. Chem. Soc., 2007, 129, 3478–3479.
Highly enantiomeric supramolecular [4+ 4] photocyclodimerization of 2-anthracenecarboxylate mediated by human serum albuminMasaki Nishijima, Takehiko Wada, Tadashi Mori, Tamara CS Pace, Cornelia Bohne*, and Yoshihisa Inoue*J. Am. Chem. Soc., 2007, 129, 3478–3479.Photoirradiation at λ > 320 nm of 2-anthracenecarboxylate bound to human serum albumin in an aqueous buffer solution at 5 °C gave syn head-to-tail cyclodimer 2 in 82% ee and anti head-to-head cyclodimer 3 in 90% ee.
@article{nishijima2007highly, title = {Highly enantiomeric supramolecular [4+ 4] photocyclodimerization of 2-anthracenecarboxylate mediated by human serum albumin}, author = {Nishijima, Masaki and Wada, Takehiko and Mori, Tadashi and Pace, Tamara CS and Bohne, Cornelia and Inoue, Yoshihisa}, journal = {J. Am. Chem. Soc.}, volume = {129}, number = {12}, pages = {3478--3479}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/ja068475z}, url = {https://doi.org/10.1021/ja068475z}, dimensions = {true}, tab = {paper}, } -
 Inherently chiral molecular clips: synthesis, chiroptical properties, and application to chiral discriminationGaku Fukuhara, Süreyya Madenci, Jolanta Polkowska, Frank Bastkowski, Frank-Gerrit Klärner, Yumi Origane*, Masayuki Kaneda, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*Chem. Eur. J., 2007, 13, 2473–2479.
Inherently chiral molecular clips: synthesis, chiroptical properties, and application to chiral discriminationGaku Fukuhara, Süreyya Madenci, Jolanta Polkowska, Frank Bastkowski, Frank-Gerrit Klärner, Yumi Origane*, Masayuki Kaneda, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*Chem. Eur. J., 2007, 13, 2473–2479.Inherently chiral molecular clips (MCs), pseudoenantiomeric anti-1 and anti-2, as well as mesoid syn-3, were synthesized by diastereodifferentiating repetitive Diels–Alder reactions of the achiral bisdienophile 6 with chiral diene 5 generated in situ from (−)-menthyl 3,4-bis(dibromomethyl)benzoate 4. These MCs were successfully separated by chiral HPLC to give optically active anti-1 and anti-2 and almost optically inactive syn-3. The structures of anti-1, anti-2, and syn-3 were assigned by high-resolution NMR and the absolute configurations of anti-1 and anti-2 were determined by the exciton-chirality method. Optically active anti-2 can serve as a chiral host. It binds the HCl adduct of D-tryptophan methyl ester (D-TrpOMe⋅HCl) 3.5 times stronger than the L-enantiomer (KD/KL=3.5).
@article{fukuhara2007inherently, title = {Inherently chiral molecular clips: synthesis, chiroptical properties, and application to chiral discrimination}, author = {Fukuhara, Gaku and Madenci, Süreyya and Polkowska, Jolanta and Bastkowski, Frank and Klärner, Frank-Gerrit and Origane, Yumi and Kaneda, Masayuki and Mori, Tadashi and Wada, Takehiko and Inoue, Yoshihisa}, journal = {Chem. Eur. J.}, volume = {13}, number = {9}, pages = {2473--2479}, year = {2007}, publisher = {Wiley Online Library}, doi = {10.1002/chem.200601585}, url = {https://doi.org/10.1002/chem.200601585}, dimensions = {true}, tab = {paper}, } -
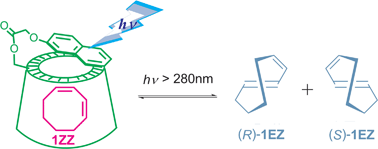 Supramolecular enantiodifferentiating photoisomerization of (Z, Z)-1, 3-cyclooctadiene included and sensitized by naphthalene-modified cyclodextrinsCheng Yang, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*New J. Chem., 2007, 31, 697–702.
Supramolecular enantiodifferentiating photoisomerization of (Z, Z)-1, 3-cyclooctadiene included and sensitized by naphthalene-modified cyclodextrinsCheng Yang, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*New J. Chem., 2007, 31, 697–702.Three naphthalene-modified cyclodextrins (CDs) 3–5 were synthesized as supramolecular chiral photosensitizing hosts for enantiodifferentiating photoisomerization of (Z,Z)-1,3-cyclooctadiene (1ZZ) to its E,Z-isomer (1EZ). In aqueous methanolic solutions, β-CD-based sensitizer 4 binds 1ZZ in its chiral cavity much more strongly than α- and γ-CD homologues 3 and 5. Accelerated, often static, fluorescence quenching of these naphthalene-modified CDs was observed upon inclusion of 1ZZ. The photoisomerization of 1ZZ mediated by 3–5 yielded enantiomeric 1EZ in modest yields. The enantiomeric excesses (ee’s) obtained with α- and β-CD-based sensitizers 3 and 4, both of which have relatively small cavities, are less sensitive to temperature, demonstrating the low-entropy nature of the α- and β-CD complexes. In contrast, increasing reaction temperature significantly diminished the product’s ee and even caused a switching of enantioselectivity upon photoisomerization sensitized by γ-CD-based 5, revealing that the entropy factor plays a crucial role in the wide cavity of γ-CD.
@article{yang2007supramolecular, title = {Supramolecular enantiodifferentiating photoisomerization of (Z, Z)-1, 3-cyclooctadiene included and sensitized by naphthalene-modified cyclodextrins}, author = {Yang, Cheng and Mori, Tadashi and Wada, Takehiko and Inoue, Yoshihisa}, journal = {New J. Chem.}, volume = {31}, number = {5}, pages = {697--702}, year = {2007}, publisher = {Royal Society of Chemistry}, doi = {10.1039/b615353d}, url = {https://doi.org/10.1039/b615353d}, dimensions = {true}, tab = {paper}, } -
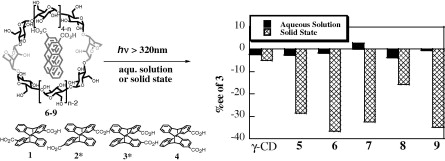 A remarkable stereoselectivity switching upon solid-state versus solution-phase enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by native and 3, 6-anhydro-γ-cyclodextrinsCheng Yang*, Masaki Nishijima, Asao Nakamura, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*Tetrahedron Lett., 2007, 48, 4357–4360.
A remarkable stereoselectivity switching upon solid-state versus solution-phase enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by native and 3, 6-anhydro-γ-cyclodextrinsCheng Yang*, Masaki Nishijima, Asao Nakamura, Tadashi Mori, Takehiko Wada, and Yoshihisa Inoue*Tetrahedron Lett., 2007, 48, 4357–4360.The enantiodifferentiating [4+4] photocyclodimerization of anthracenecarboxylic acid (AC) mediated by native, mono- and di-3,6-anhydro-γ-cyclodextrins was investigated in both aqueous solution and solid-state. The solid-state photolyses gave inherently disfavored head-to-head photodimers in much higher chemical and optical yields than in the aqueous solution.
@article{yang2007remarkable, title = {A remarkable stereoselectivity switching upon solid-state versus solution-phase enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by native and 3, 6-anhydro-γ-cyclodextrins}, author = {Yang, Cheng and Nishijima, Masaki and Nakamura, Asao and Mori, Tadashi and Wada, Takehiko and Inoue, Yoshihisa}, journal = {Tetrahedron Lett.}, volume = {48}, number = {25}, pages = {4357--4360}, year = {2007}, publisher = {Elsevier}, doi = {10.1016/j.tetlet.2007.04.104}, url = {https://doi.org/10.1016/j.tetlet.2007.04.104}, dimensions = {true}, tab = {paper}, } -
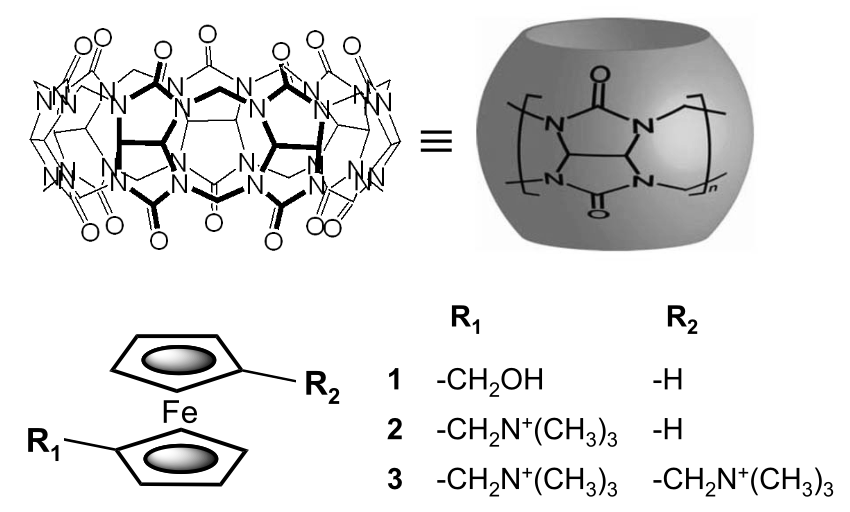 A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy–entropy compensationMikhail V Rekharsky, Tadashi Mori, Cheng Yang, Young Ho Ko, N Selvapalam, Hyunuk Kim, David Sobransingh, Angel E Kaifer*, Simin Liu, Lyle Isaacs*, Wei Chen, Sarvin Moghaddam, Michael K Gilson*, Kimoon Kim*, and Yoshihisa Inoue*Proc. Natl. Acad. Sci., 2007, 104, 20737–20742.
A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy–entropy compensationMikhail V Rekharsky, Tadashi Mori, Cheng Yang, Young Ho Ko, N Selvapalam, Hyunuk Kim, David Sobransingh, Angel E Kaifer*, Simin Liu, Lyle Isaacs*, Wei Chen, Sarvin Moghaddam, Michael K Gilson*, Kimoon Kim*, and Yoshihisa Inoue*Proc. Natl. Acad. Sci., 2007, 104, 20737–20742.The molecular host cucurbit[7]uril forms an extremely stable inclusion complex with the dicationic ferrocene derivative bis(trimethylammoniomethyl)ferrocene in aqueous solution. The equilibrium association constant for this host-guest pair is 3 × 1015 M−1 (Kd = 3 × 10−16 M), equivalent to that exhibited by the avidin–biotin pair. Although purely synthetic systems with larger association constants have been reported, the present one is unique because it does not rely on polyvalency. Instead, it achieves its extreme affinity by overcoming the compensatory enthalpy–entropy relationship usually observed in supramolecular complexes. Its disproportionately low entropic cost is traced to extensive host desolvation and to the rigidity of both the host and the guest.
@article{rekharsky2007synthetic, title = {A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy--entropy compensation}, author = {Rekharsky, Mikhail V and Mori, Tadashi and Yang, Cheng and Ko, Young Ho and Selvapalam, N and Kim, Hyunuk and Sobransingh, David and Kaifer, Angel E and Liu, Simin and Isaacs, Lyle and Chen, Wei and Moghaddam, Sarvin and Gilson, Michael K and Kim, Kimoon and Inoue, Yoshihisa}, journal = {Proc. Natl. Acad. Sci.}, volume = {104}, number = {52}, pages = {20737--20742}, year = {2007}, publisher = {National Academy of Sciences}, doi = {10.1073/pnas.0706407105}, url = {https://doi.org/10.1073/pnas.0706407105}, dimensions = {true}, tab = {paper}, } -
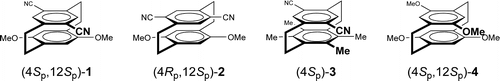 Quantum chemical study on the circular dichroism spectra and specific rotation of donor- acceptor cyclophanesTadashi Mori*, Yoshihisa Inoue, and Stefan Grimme*J. Phys. Chem. A, 2007, 111, 7995–8006.
Quantum chemical study on the circular dichroism spectra and specific rotation of donor- acceptor cyclophanesTadashi Mori*, Yoshihisa Inoue, and Stefan Grimme*J. Phys. Chem. A, 2007, 111, 7995–8006.The structures of donor,acceptor-substituted cyclophanes were optimized by DFT and MP2 methods and compared with the X-ray crystallographic structures. The electronic circular dichroism (CD) spectra of these chiral cyclophanes were simulated by time dependent density functional theory (TD−DFT) with several functionals including different amounts of “exact” Hartree−Fock exchange. The experimental oscillator and rotatory strengths were best reproduced by the BH-LYP/TZV2P method. The specific rotation and vibrational circular dichroism (VCD) spectra were also calculated at the BH-LYP/aug-cc-pVDZ and B3-LYP/6-31G(d) levels, respectively, and compared with the experimental data. Better performance was obtained with the ECD, rather than the specific rotation or the VCD spectral calculations in view of the computation time and accuracy for the determination of absolute configuration (AC). The exciton coupling model can be applied only for the cyclophanes without CT-character. However, the split pattern found in the experiment does not appear to originate from a simple two-transition coupling, indicating that this method should be applied with caution to the AC determination. This conclusion was supported by the TD−DFT investigations of the transition moments and the roles of excited-state electronic configuration associated with these split bands. Cyclophanes with donor−acceptor interactions showed Cotton effects at the CT band and couplets at the 1La and 1Lb bands. Although the degree of charge transfer between the rings is very small, as revealed by a Mulliken−Hash analysis, the split Cotton effects are due to a large separation in energy of the donor and acceptor orbitals. The effect of the distance and angle between the donor and acceptor moieties in model (intermolecular) CT complexes on the calculated CD spectra was also studied and compared with those obtained for various paracyclophanes.
@article{mori2007quantum, title = {Quantum chemical study on the circular dichroism spectra and specific rotation of donor- acceptor cyclophanes}, author = {Mori, Tadashi and Inoue, Yoshihisa and Grimme, Stefan}, journal = {J. Phys. Chem. A}, volume = {111}, number = {32}, pages = {7995--8006}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/jp073596m}, url = {https://doi.org/10.1021/jp073596m}, dimensions = {true}, tab = {paper}, } -
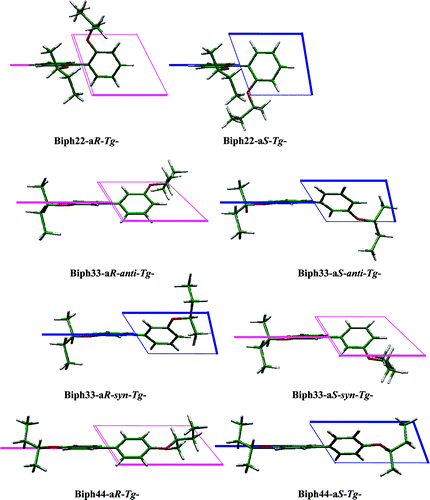 Experimental and Theoretical Study of the CD Spectra and Conformational Properties of Axially Chiral 2, 2 ‘-, 3, 3 ‘-, and 4, 4 ‘-Biphenol EthersTadashi Mori*, Yoshihisa Inoue, and Stefan GrimmeJ. Phys. Chem. A, 2007, 111, 4222–4234.
Experimental and Theoretical Study of the CD Spectra and Conformational Properties of Axially Chiral 2, 2 ‘-, 3, 3 ‘-, and 4, 4 ‘-Biphenol EthersTadashi Mori*, Yoshihisa Inoue, and Stefan GrimmeJ. Phys. Chem. A, 2007, 111, 4222–4234.@article{mori2007experimental, title = {Experimental and Theoretical Study of the CD Spectra and Conformational Properties of Axially Chiral 2, 2 ‘-, 3, 3 ‘-, and 4, 4 ‘-Biphenol Ethers}, author = {Mori, Tadashi and Inoue, Yoshihisa and Grimme, Stefan}, journal = {J. Phys. Chem. A}, volume = {111}, number = {20}, pages = {4222--4234}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/jp071709w}, url = {https://doi.org/10.1021/jp071709w}, dimensions = {true}, tab = {paper}, } -
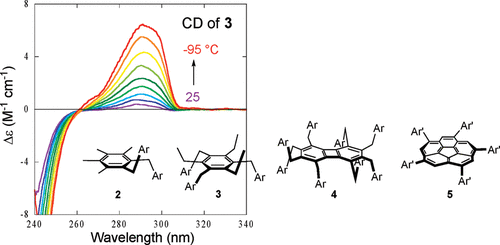 A combined experimental and theoretical study on the conformation of multiarmed chiral aryl ethersTadashi Mori*, Stefan Grimme, and Yoshihisa InoueJ. Org. Chem., 2007, 72, 6998–7010.
A combined experimental and theoretical study on the conformation of multiarmed chiral aryl ethersTadashi Mori*, Stefan Grimme, and Yoshihisa InoueJ. Org. Chem., 2007, 72, 6998–7010.Four series of multiarmed chiral aryl ethers carrying two, three, five, or eight side-chains on a variety of aromatic core molecules (2−5) were prepared. The structure and conformation of 2 and 3 (in the solid state) were determined by the X-ray crystallographic analyses. While a pair of alternated (anti) conformers (i.e, up−down and down−up) were found in the crystal of 2, three side-arms in 3 were aligned in the same direction to give a C3-symmetric syn-conformation. Examinations by dispersion-corrected density functional (DFT-D) calculations revealed that two out of six anti- and two out of four syn-conformers of 2 are energetically most important. Two calculated structures of anti-conformers are in good agreement with those found in the solid state by X-ray analysis. Similarly, relevant conformations of syn-3, fully alternated 4, and C5-symmetric 5 were optimized at the DFT-D-B-LYP/TZVP level. The structure and conformation of the side-arms in 2−5 in solution were further studied by temperature dependent 1H NMR and UV−vis spectroscopy. In addition, comparative experimental and theoretical CD spectral studies were carried out in order to elucidate the contribution of the thermodynamically less-stable minor isomers in solution. The CD spectral changes observed for 2 and 3 at varying temperatures were quite different, while the parent chiral arene 1, as well as 4 and 5, only showed an increased intensity of the negative Cotton effect for the 1Lb band. The latter behavior is readily accounted for in terms of the conformational freezing of the chiral groups at low temperatures. The unusual CD spectral behavior observed for 2 and 3 was rationalized by the conformational alteration of the side-arms. Because of attractive van der Waals interactions between the aromatic units of the arms in nonpolar solvents, the syn-conformations become gradually more important for 2 at low temperatures, which eventually results in a weak positive Cotton effect for the 1Lb band. This was also supported by the SCS-MP2/TZVPP single-point energy calculations for the relevant conformers of 2. For 3, the contribution of the C3-symmetrical conformer becomes more important than the less-symmetrical isomers at low temperatures. The conformations of 2 and 3 in their excited states as well as in the oxidized states were also examined.
@article{mori2007combined, title = {A combined experimental and theoretical study on the conformation of multiarmed chiral aryl ethers}, author = {Mori, Tadashi and Grimme, Stefan and Inoue, Yoshihisa}, journal = {J. Org. Chem.}, volume = {72}, number = {18}, pages = {6998--7010}, year = {2007}, publisher = {ACS Publications}, doi = {10.1021/jo071216n}, url = {https://doi.org/10.1021/jo071216n}, dimensions = {true}, tab = {paper}, }